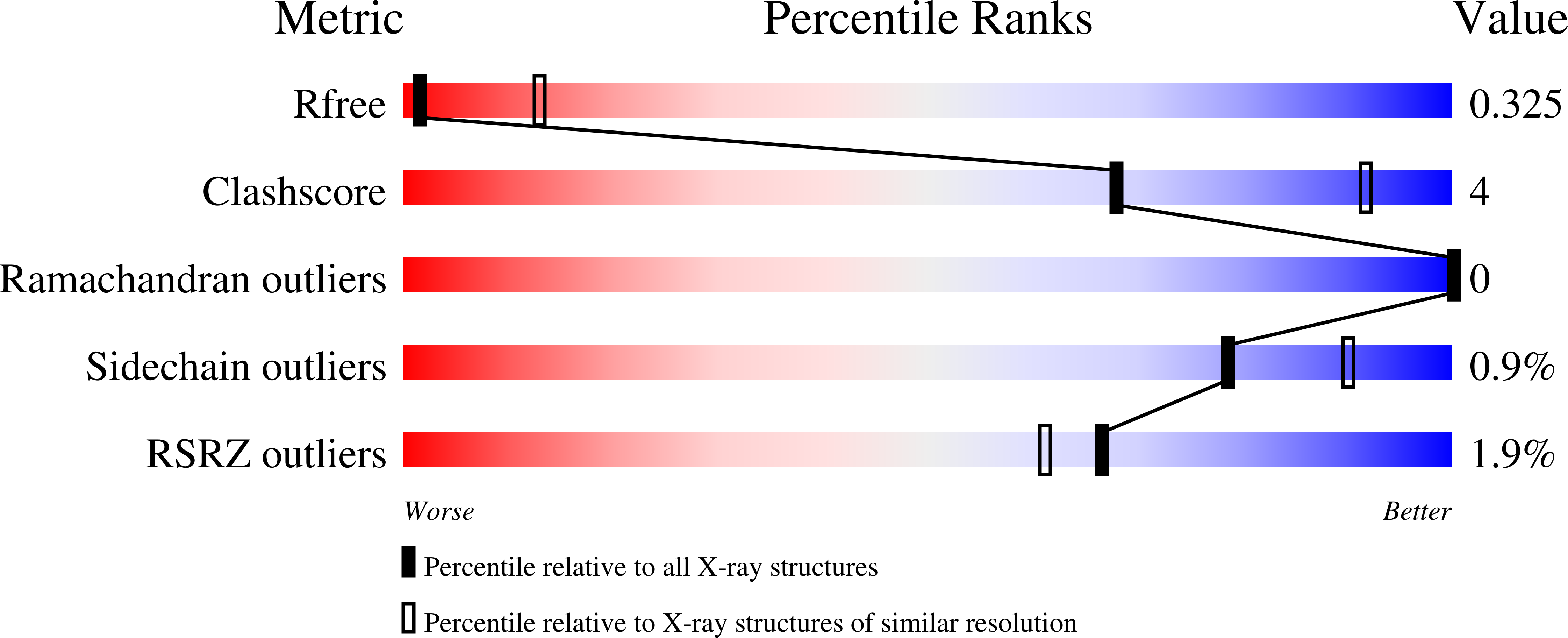
Structure of a photosynthetic reaction centre determined by serial femtosecond crystallography.
Johansson, L.C., Arnlund, D., Katona, G., White, T.A., Barty, A., DePonte, D.P., Shoeman, R.L., Wickstrand, C., Sharma, A., Williams, G.J., Aquila, A., Bogan, M.J., Caleman, C., Davidsson, J., Doak, R.B., Frank, M., Fromme, R., Galli, L., Grotjohann, I., Hunter, M.S., Kassemeyer, S., Kirian, R.A., Kupitz, C., Liang, M., Lomb, L., Malmerberg, E., Martin, A.V., Messerschmidt, M., Nass, K., Redecke, L., Seibert, M.M., Sjohamn, J., Steinbrener, J., Stellato, F., Wang, D., Wahlgren, W.Y., Weierstall, U., Westenhoff, S., Zatsepin, N.A., Boutet, S., Spence, J.C., Schlichting, I., Chapman, H.N., Fromme, P., Neutze, R.(2013) Nat Commun 4: 2911-2911
- PubMed: 24352554
- DOI: https://doi.org/10.1038/ncomms3911
- Primary Citation of Related Structures:
4CAS - PubMed Abstract:
Serial femtosecond crystallography is an X-ray free-electron-laser-based method with considerable potential to have an impact on challenging problems in structural biology. Here we present X-ray diffraction data recorded from microcrystals of the Blastochloris viridis photosynthetic reaction centre to 2.8 Å resolution and determine its serial femtosecond crystallography structure to 3.5 Å resolution. Although every microcrystal is exposed to a dose of 33 MGy, no signs of X-ray-induced radiation damage are visible in this integral membrane protein structure.
Organizational Affiliation:
Department of Chemistry and Molecular Biology, University of Gothenburg, 405 30 Gothenburg, Sweden.