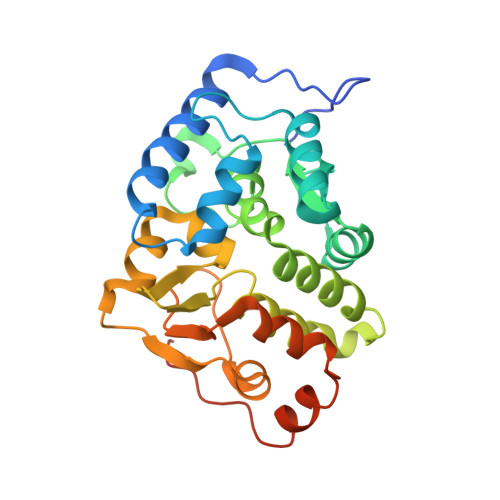
Structural and Thermodynamic Insight Into Phenylalanine Hydroxylase from the Human Pathogen Legionella Pneumophila.
Leiros, H.S., Flydal, M.I., Martinez, A.(2013) FEBS Open Bio 3: 370
- PubMed: 24251098
- DOI: https://doi.org/10.1016/j.fob.2013.08.006
- Primary Citation of Related Structures:
4BPT - PubMed Abstract:
Phenylalanine hydroxylase from Legionella pneumophila (lpPAH) has a major functional role in the synthesis of the pigment pyomelanin, which is a potential virulence factor. We present here the crystal structure of lpPAH, which is a dimeric enzyme that shows high thermostability, with a midpoint denaturation temperature of 79 °C, and low substrate affinity. The structure revealed a dimerization motif that includes ionic interactions and a hydrophobic core, composed of both β-structure and a C-terminal region, with the specific residues (P255, P256, Y257 and F258) interacting with the same residues from the adjacent subunit within the dimer. This unique dimerization interface, together with a number of aromatic clusters, appears to contribute to the high thermal stability of lpPAH. The crystal structure also explains the increased aggregation of the enzyme in the presence of salt. Moreover, the low affinity for substrate l-Phe could be explained from three consecutive glycine residues (G181, 182, 183) located at the substrate-binding site. This is the first structure of a dimeric bacterial PAH and provides a framework for interpreting the molecular and kinetic properties of lpPAH and for further investigating the regulation of the enzyme.
Organizational Affiliation:
The Norwegian Structural Biology Centre (NorStruct), Department of Chemistry, University of Tromsø, N-9037 Tromsø, Norway.