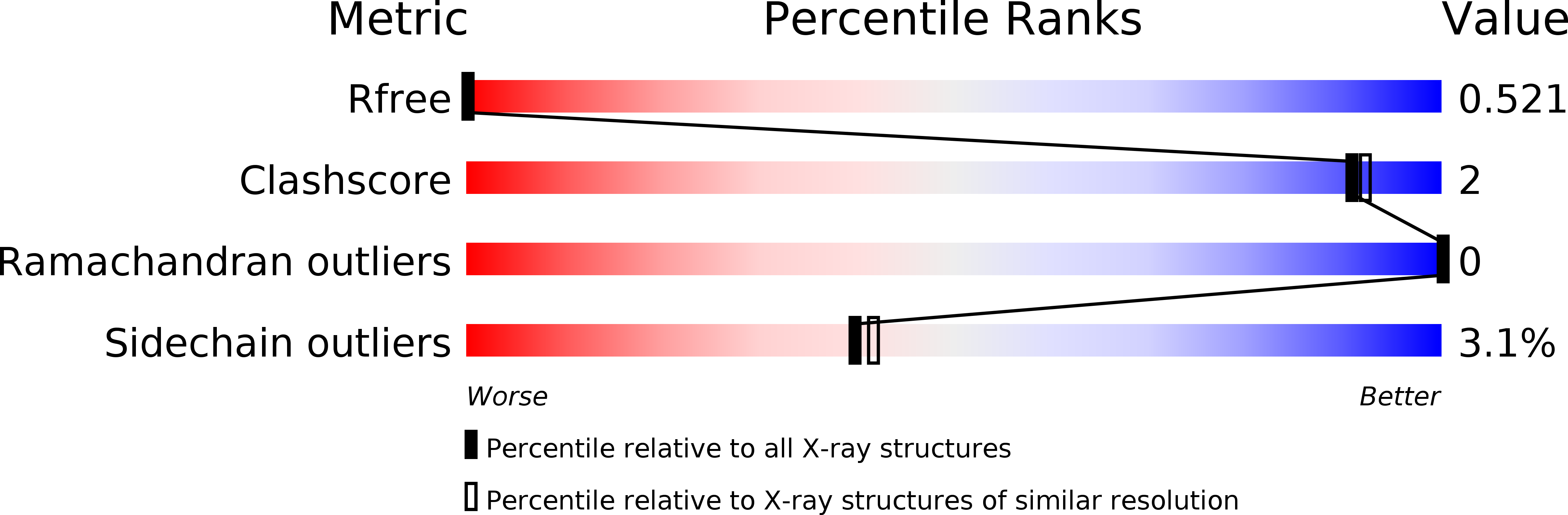Multivalent Interactions of the Sumo-Interaction Motifs in the Ring-Finger Protein 4 (Rnf4) Determine the Specificity for Chains of the Small Ubiquitin-Related Modifier (Sumo).
Keusekotten, K., Bade, V.N., Meyer-Teschendorf, K., Sriramachandran, A.M., Fischer-Schrader, K., Krause, A., Horst, C., Schwarz, G., Hofmann, K., Dohmen, R.J., Praefcke, G.J.(2014) Biochem J 457: 207
- PubMed: 24151981
- DOI: https://doi.org/10.1042/BJ20130753
- Primary Citation of Related Structures:
4BKG - PubMed Abstract:
RNF4 (RING finger protein 4) is a STUbL [SUMO (small ubiquitin-related modifier)-targeted ubiquitin ligase] controlling PML (promyelocytic leukaemia) nuclear bodies, DNA double strand break repair and other nuclear functions. In the present paper, we describe that the sequence and spacing of the SIMs (SUMO-interaction motifs) in RNF4 regulate the avidity-driven recognition of substrate proteins carrying SUMO chains of variable length.
Organizational Affiliation:
†Institute for Genetics, University of Cologne, 50674 Köln, Germany.