A glutathione-dependent detoxification system is required for formaldehyde resistance and optimal survival of Neisseria meningitidis in biofilms.
Chen, N.H., Counago, R.M., Djoko, K.Y., Jennings, M.P., Apicella, M.A., Kobe, B., McEwan, A.G.(2013) Antioxid Redox Signal 18: 743-755
- PubMed: 22937752
- DOI: https://doi.org/10.1089/ars.2012.4749
- Primary Citation of Related Structures:
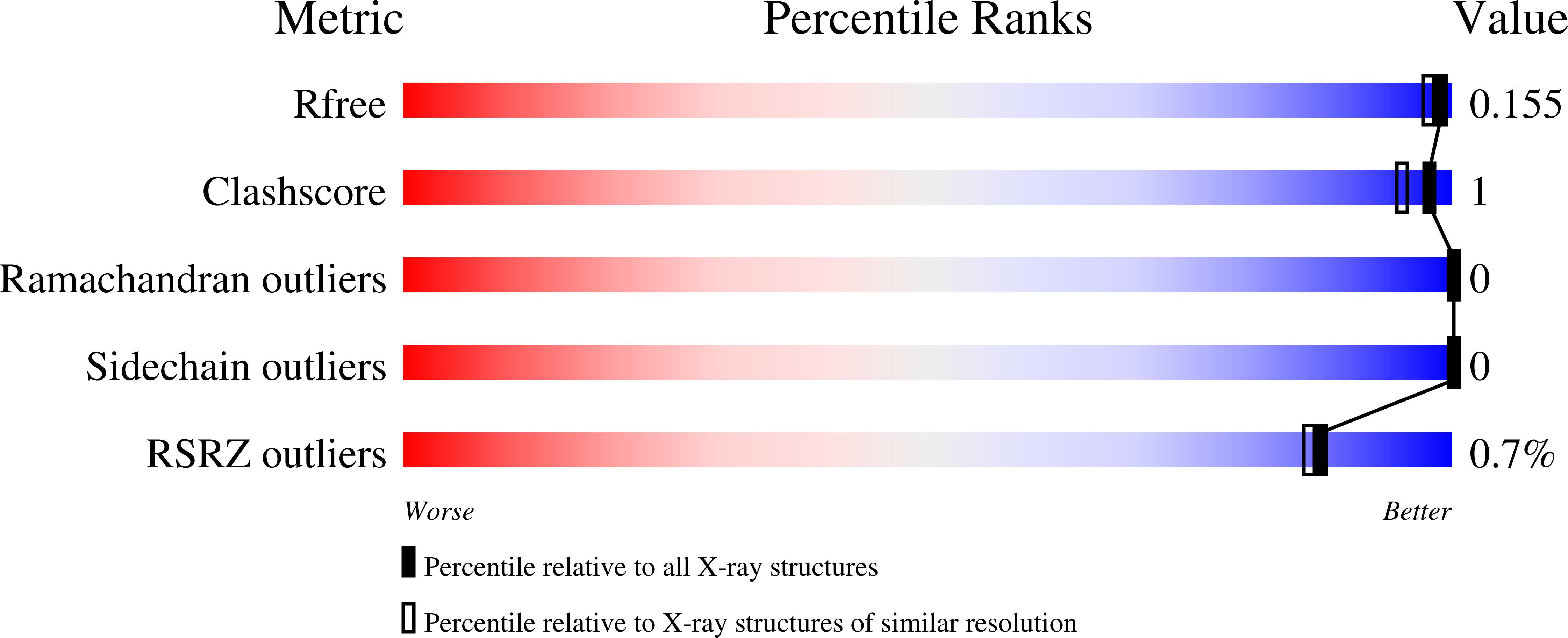
4B6G - PubMed Abstract:
The glutathione-dependent AdhC-EstD formaldehyde detoxification system is found in eukaryotes and prokaryotes. It is established that it confers protection against formaldehyde that is produced from environmental sources or methanol metabolism. Thus, its presence in the human host-adapted bacterial pathogen Neisseria meningitidis is intriguing. This work defined the biological function of this system in the meningococcus using phenotypic analyses of mutants linked to biochemical and structural characterization of purified enzymes. We demonstrated that mutants in the adhC and/or estD were sensitive to killing by formaldehyde. Inactivation of adhC and/or estD also led to a loss of viability in biofilm communities, even in the absence of exogenous formaldehyde. Detailed biochemical and structural analyses of the esterase component demonstrated that S-formylglutathione was the only biologically relevant substrate for EstD. We further showed that an absolutely conserved cysteine residue was covalently modified by S-glutathionylation. This leads to inactivation of EstD. The results provide several conceptual innovations. They provide a new insight into formaldehyde detoxification in bacteria that do not generate formaldehyde during the catabolism of methanol. Our results also indicate that the conserved cysteine, found in all EstD enzymes from humans to microbes, is a site of enzyme regulation, probably via S-glutathionylation. The adhc-estD system protects against formaldehyde produced during endogenous metabolism.
Organizational Affiliation:
Australian Centre for Infectious Disease Research, School of Chemistry and Molecular Biosciences, Faculty of Science, The University of Queensland, St. Lucia, Australia.