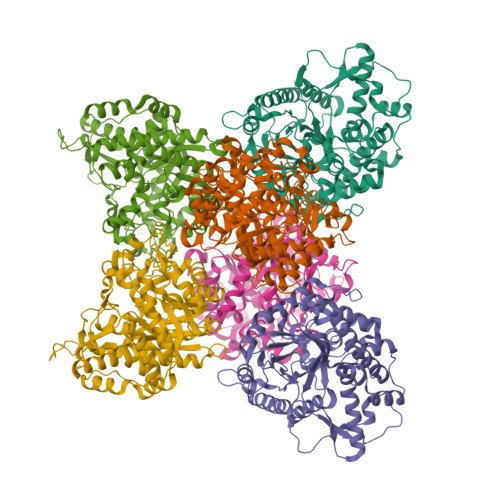
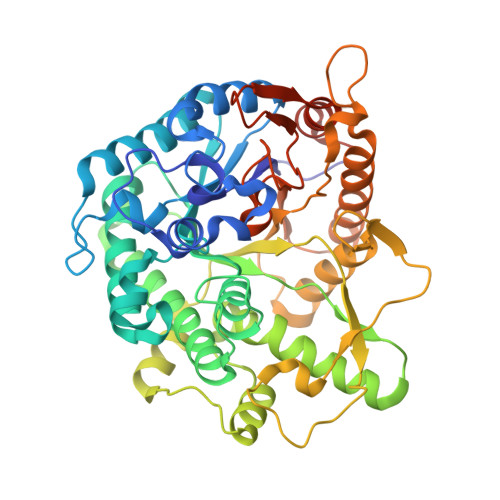
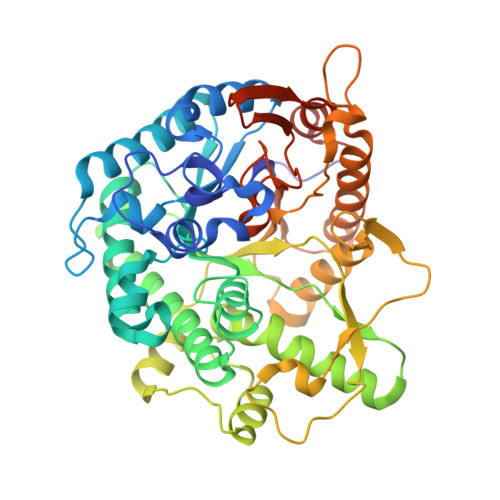
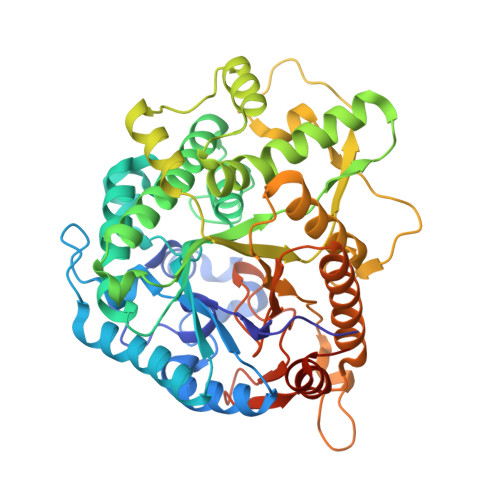
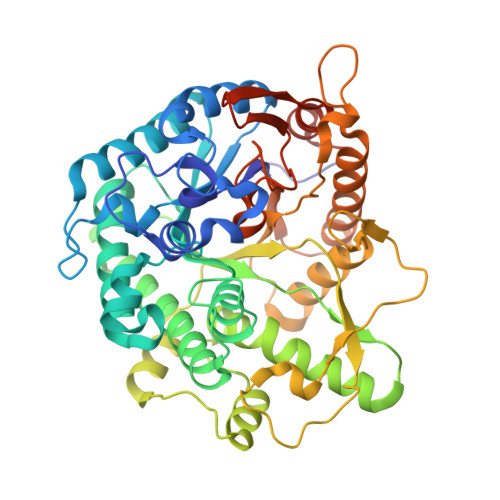
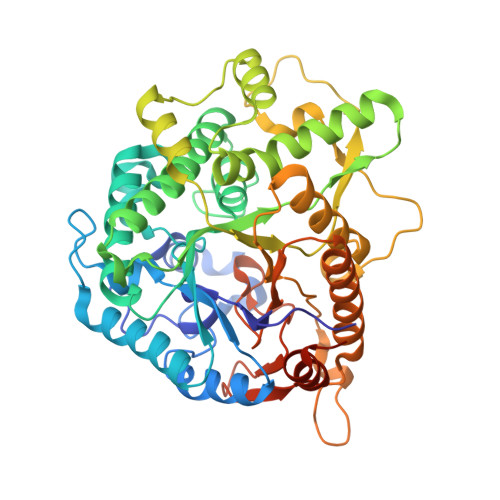
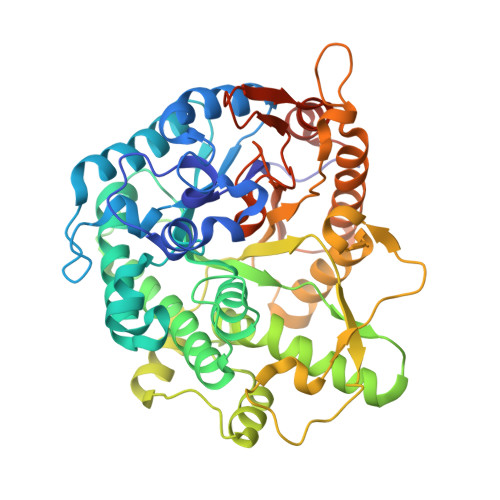
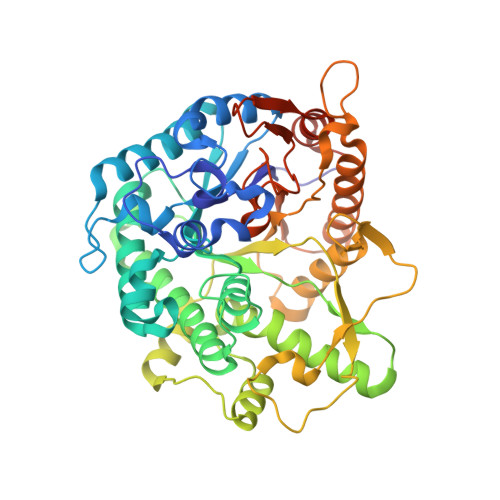
Structure and Activity of the Streptococcus Pyogenes Family Gh1 6-Phospho Beta-Glycosidase Spy1599
Stepper, J., Dabin, J., Ekloef, J.M., Thongpoo, P., Kongsaeree, P.T., Taylor, E.J., Turkenburg, J.P., Brumer, H., Davies, G.J.(2013) Acta Crystallogr D Biol Crystallogr 69: 16
- PubMed: 23275159
- DOI: https://doi.org/10.1107/S0907444912041005
- Primary Citation of Related Structures:
4B3K, 4B3L - PubMed Abstract:
The group A streptococcus Streptococcus pyogenes is the causative agent of a wide spectrum of invasive infections, including necrotizing fasciitis, scarlet fever and toxic shock syndrome. In the context of its carbohydrate chemistry, it is interesting that S. pyogenes (in this work strain M1 GAS SF370) displays a spectrum of oligosaccharide-processing enzymes that are located in close proximity on the genome but that the in vivo function of these proteins remains unknown. These proteins include different sugar transporters (SPy1593 and SPy1595), both GH125 α-1,6- and GH38 α-1,3-mannosidases (SPy1603 and SPy1604), a GH84 β-hexosaminidase (SPy1600) and a putative GH2 β-galactosidase (SPy1586), as well as SPy1599, a family GH1 `putative β-glucosidase'. Here, the solution of the three-dimensional structure of SPy1599 in a number of crystal forms complicated by unusual crystallographic twinning is reported. The structure is a classical (β/α)(8)-barrel, consistent with CAZy family GH1 and other members of the GH-A clan. SPy1599 has been annotated in sequence depositions as a β-glucosidase (EC 3.2.1.21), but no such activity could be found; instead, three-dimensional structural overlaps with other enzymes of known function suggested that SPy1599 contains a phosphate-binding pocket in the active site and has possible 6-phospho-β-glycosidase activity. Subsequent kinetic analysis indeed showed that SPy1599 has 6-phospho-β-glucosidase (EC 3.2.1.86) activity. These data suggest that SPy1599 is involved in the intracellular degradation of 6-phosphoglycosides, which are likely to originate from import through one of the organism's many phosphoenolpyruvate phosphotransfer systems (PEP-PTSs).
Organizational Affiliation:
Department of Chemistry, University of York, Heslington, York YO10 5DD, England.