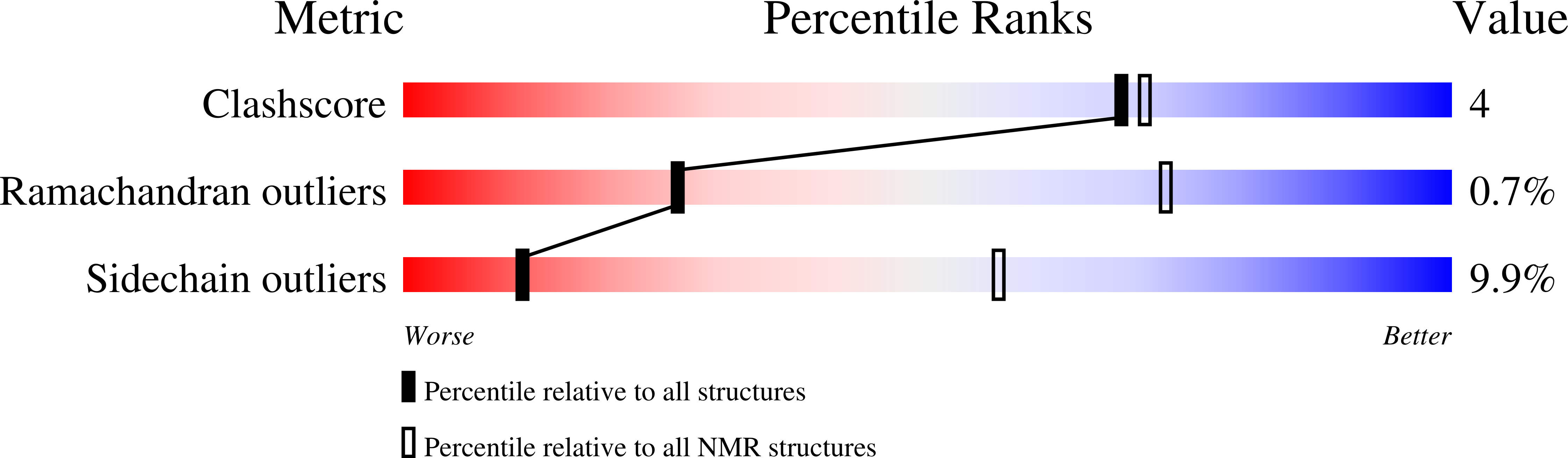Structure of the Sgt2/Get5 Complex Provides Insights Into Get-Mediated Targeting of Tail-Anchored Membrane Proteins
Simon, A.C., Simpson, P.J., Goldstone, R.M., Krysztofinska, E.M., Murray, J.W., High, S., Isaacson, R.L.(2013) Proc Natl Acad Sci U S A 110: 1327
- PubMed: 23297211
- DOI: https://doi.org/10.1073/pnas.1207518110
- Primary Citation of Related Structures:
4A20, 4ASV, 4ASW - PubMed Abstract:
Small, glutamine-rich, tetratricopeptide repeat protein 2 (Sgt2) is the first known port of call for many newly synthesized tail-anchored (TA) proteins released from the ribosome and destined for the GET (Guided Entry of TA proteins) pathway. This leads them to the residential membrane of the endoplasmic reticulum via an alternative to the cotranslational, signal recognition particle-dependent mechanism that their topology denies them. In yeast, the first stage of the GET pathway involves Sgt2 passing TA proteins on to the Get4/Get5 complex through a direct interaction between the N-terminal (NT) domain of Sgt2 and the ubiquitin-like (UBL) domain of Get5. Here we characterize this interaction at a molecular level by solving both a solution structure of Sgt2_NT, which adopts a unique helical fold, and a crystal structure of the Get5_UBL. Furthermore, using reciprocal chemical shift perturbation data and experimental restraints, we solve a structure of the Sgt2_NT/Get5_UBL complex, validate it via site-directed mutagenesis, and empirically determine its stoichiometry using relaxation experiments and isothermal titration calorimetry. Taken together, these data provide detailed structural information about the interaction between two key players in the coordinated delivery of TA protein substrates into the GET pathway.
Organizational Affiliation:
Division of Molecular Biosciences, Imperial College London, London SW7 2AZ, United Kingdom.