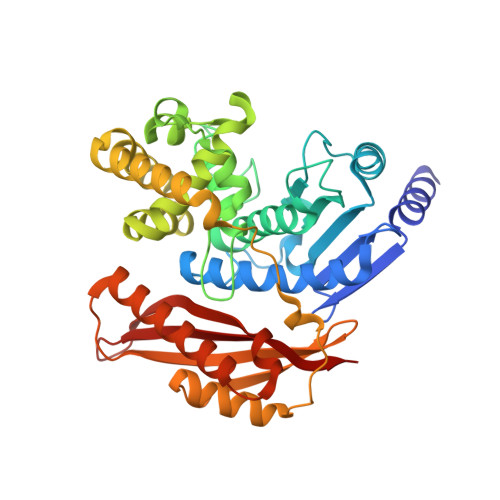
Structural Basis for Activity Regulation and Substrate Preference of Clostridial Collagenases G, H, and T.
Eckhard, U., Schonauer, E., Brandstetter, H.(2013) J Biol Chem 288: 20184
- PubMed: 23703618
- DOI: https://doi.org/10.1074/jbc.M112.448548
- Primary Citation of Related Structures:
4AQO, 4AR1, 4AR8, 4AR9, 4ARE, 4ARF - PubMed Abstract:
Clostridial collagenases are among the most efficient enzymes to degrade by far the most predominant protein in the biosphere. Here we present crystal structures of the peptidases of three clostridial collagenase isoforms (ColG, ColH, and ColT). The comparison of unliganded and liganded structures reveals a quaternary subdomain dynamics. In the unliganded ColH structure, this globular dynamics is modulated by an aspartate switch motion that binds to the catalytic zinc. We further identified a calcium binding site in proximity to the catalytic zinc. Both ions are required for full activity, explaining why calcium critically affects the enzymatic activity of clostridial collagenases. Our studies further reveal that loops close to the active site thus serve as characteristic substrate selectivity filter. These elements explain the distinct peptidolytic and collagenolytic activities of these enzymes and provide a rational framework to engineer collagenases with customized substrate specificity as well as for inhibitor design.
Organizational Affiliation:
Division of Structural Biology, Department of Molecular Biology, University of Salzburg, Billrothstrasse 11, A-5020 Salzburg, Austria.