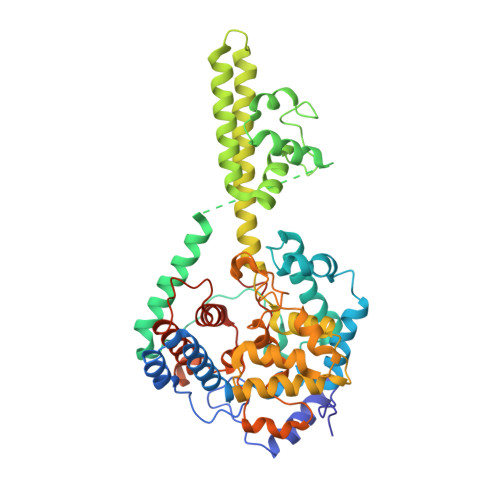
Structure of Crimean-Congo Haemorraghic Fever Virus Nucleoprotein: Superhelical Homo-Oligomers and the Role of Caspase-3 Cleavage.
Wang, Y., Dutta, S., Karlberg, H., Devignot, S., Weber, F., Hao, Q., Tan, Y.J., Mirazimi, A., Kotaka, M.(2012) J Virol 86: 12294
- PubMed: 22951837
- DOI: https://doi.org/10.1128/JVI.01627-12
- Primary Citation of Related Structures:
4AQF, 4AQG - PubMed Abstract:
Crimean-Congo hemorrhagic fever, a severe hemorrhagic disease found throughout Africa, Europe, and Asia, is caused by the tick-borne Crimean-Congo hemorrhagic fever virus (CCHFV). CCHFV is a negative-sense single-stranded RNA (ssRNA) virus belonging to the Nairovirus genus of the Bunyaviridae family. Its genome of three single-stranded RNA segments is encapsidated by the nucleocapsid protein (CCHFV N) to form the ribonucleoprotein complex. This ribonucleoprotein complex is required during replication and transcription of the viral genomic RNA. Here, we present the crystal structures of the CCHFV N in two distinct forms, an oligomeric form comprised of double antiparallel superhelices and a monomeric form. The head-to-tail interaction of the stalk region of one CCHFV N subunit with the base of the globular body of the adjacent subunit stabilizes the helical organization of the oligomeric form of CCHFV N. It also masks the conserved caspase-3 cleavage site present at the tip of the stalk region from host cell caspase-3 interaction and cleavage. By incubation with primer-length ssRNAs, we also obtained the crystal structure of CCHFV N in its monomeric form, which is similar to a recently published structure. The conformational change of CCHFV N upon deoligomerization results in the exposure of the caspase-3 cleavage site and subjects CCHFV N to caspase-3 cleavage. Mutations of this cleavage site inhibit cleavage by caspase-3 and result in enhanced viral polymerase activity. Thus, cleavage of CCHFV N by host cell caspase-3 appears to be crucial for controlling viral RNA synthesis and represents an important host defense mechanism against CCHFV infection.
Organizational Affiliation:
Department of Physiology, Li Ka Shing Faculty of Medicine, University of Hong Kong, Pokfulam, Hong Kong SAR, China.