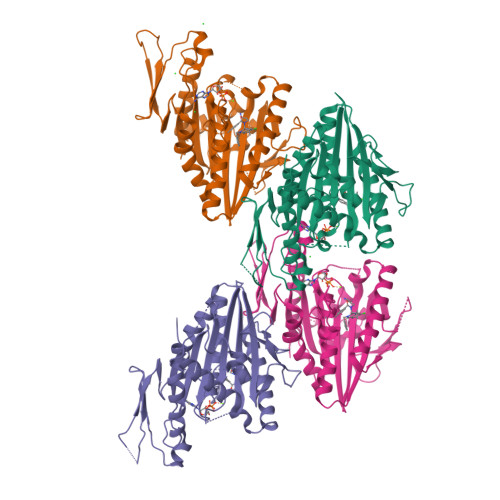
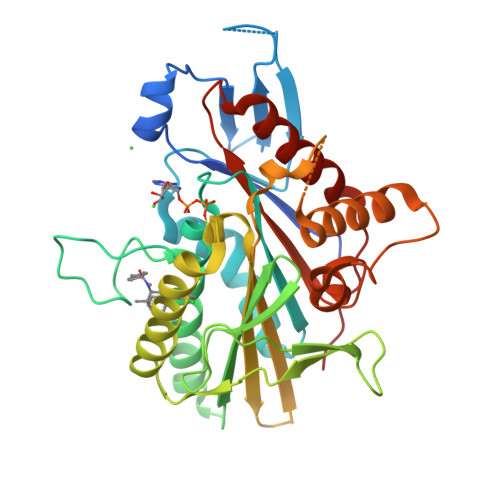
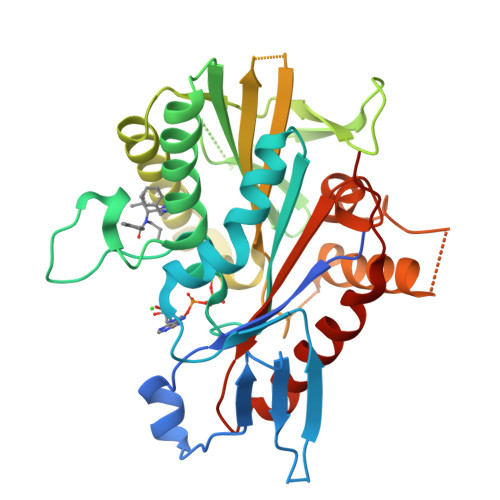
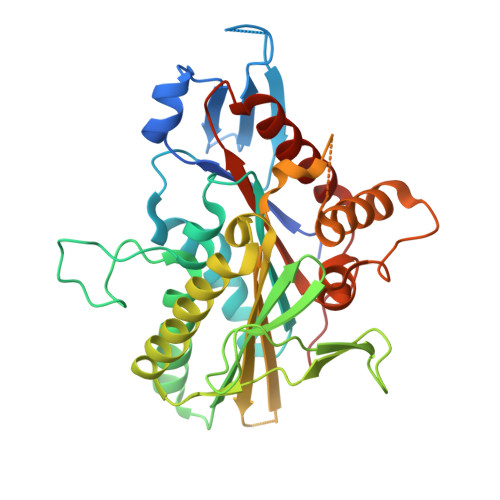
The Structure of the Ternary Eg5-Adp-Ispinesib Complex
Talapatra, S.K., Schuettelkopf, A.W., Kozielski, F.(2012) Acta Crystallogr D Biol Crystallogr 68: 1311
- PubMed: 22993085
- DOI: https://doi.org/10.1107/S0907444912027965
- Primary Citation of Related Structures:
4AP0 - PubMed Abstract:
The human kinesin Eg5 is responsible for bipolar spindle formation during early mitosis. Inhibition of Eg5 triggers the formation of monoastral spindles, leading to mitotic arrest that eventually causes apoptosis. There is increasing evidence that Eg5 constitutes a potential drug target for the development of cancer chemotherapeutics. The most advanced Eg5-targeting agent is ispinesib, which exhibits potent antitumour activity and is currently in multiple phase II clinical trials. In this study, the crystal structure of the Eg5 motor domain in complex with ispinesib, supported by kinetic and thermodynamic binding data, is reported. Ispinesib occupies the same induced-fit pocket in Eg5 as other allosteric inhibitors, making extensive hydrophobic interactions with the protein. The data for the Eg5-ADP-ispinesib complex suffered from pseudo-merohedral twinning and revealed translational noncrystallographic symmetry, leading to challenges in data processing, space-group assignment and structure solution as well as in refinement. These complications may explain the lack of available structural information for this important agent and its analogues. The present structure represents the best interpretation of these data based on extensive data-reduction, structure-solution and refinement trials.
Organizational Affiliation:
Molecular Motor Laboratory, The Beatson Institute for Cancer Research, Garscube Estate, Switchback Road, Bearsden, Glasgow G61 1BD, Scotland, UK. s.talapatra@beatson.gla.ac.uk