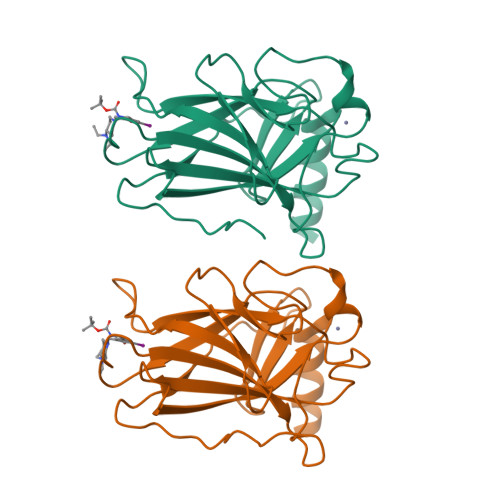
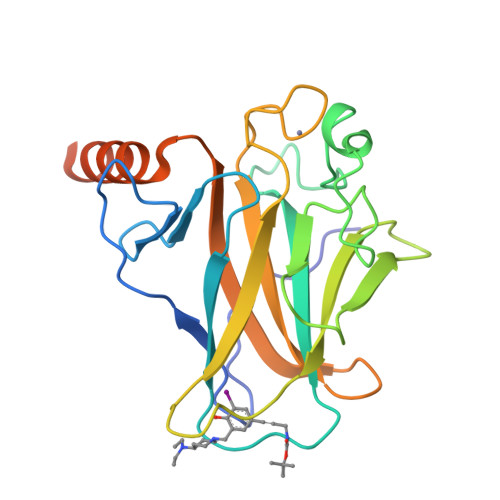
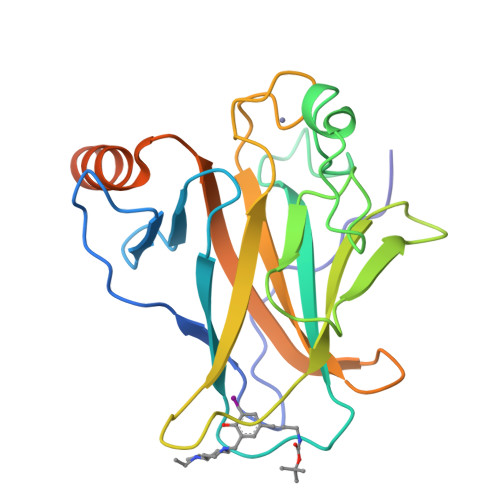
Halogen-Enriched Fragment Libraries as Leads for Drug Rescue of Mutant P53.
Wilcken, R., Liu, X., Zimmermann, M.O., Rutherford, T.J., Fersht, A.R., Joerger, A.C., Boeckler, F.M.(2012) J Am Chem Soc 134: 6810
- PubMed: 22439615
- DOI: https://doi.org/10.1021/ja301056a
- Primary Citation of Related Structures:
4AGL, 4AGM, 4AGN, 4AGO, 4AGP, 4AGQ - PubMed Abstract:
The destabilizing p53 cancer mutation Y220C creates a druggable surface crevice. We developed a strategy exploiting halogen bonding for lead discovery to stabilize the mutant with small molecules. We designed halogen-enriched fragment libraries (HEFLibs) as starting points to complement classical approaches. From screening of HEFLibs and subsequent structure-guided design, we developed substituted 2-(aminomethyl)-4-ethynyl-6-iodophenols as p53-Y220C stabilizers. Crystal structures of their complexes highlight two key features: (i) a central scaffold with a robust binding mode anchored by halogen bonding of an iodine with a main-chain carbonyl and (ii) an acetylene linker, enabling the targeting of an additional subsite in the crevice. The best binders showed induction of apoptosis in a human cancer cell line with homozygous Y220C mutation. Our structural and biophysical data suggest a more widespread applicability of HEFLibs in drug discovery.
Organizational Affiliation:
Laboratory for Molecular Design and Pharmaceutical Biophysics, Department of Pharmaceutical and Medicinal Chemistry, Institute of Pharmacy, Eberhard-Karls-University Tuebingen, Auf der Morgenstelle 8, 72076 Tuebingen, Germany.