Structural and Functional Characterization of an Smc-Like Protein Recn: New Insights Into Double-Strand Break Repair.
Pellegrino, S., Radzimanowski, J., De Sanctis, D., Erba, E.B., Mcsweeney, S., Timmins, J.(2012) Structure 20: 2076
- PubMed: 23085075
- DOI: https://doi.org/10.1016/j.str.2012.09.010
- Primary Citation of Related Structures:
4ABX, 4ABY, 4AD8 - PubMed Abstract:
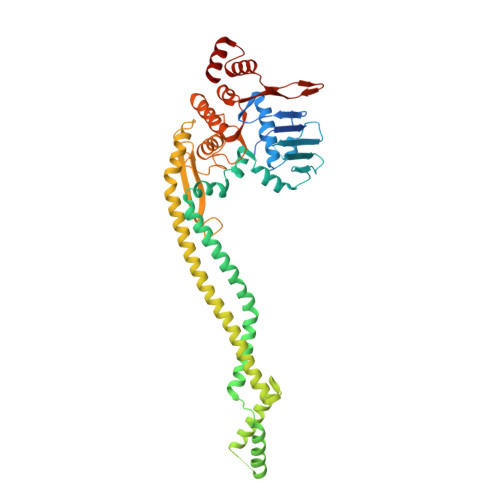
Repair of DNA double-strand breaks (DSBs) is essential for cell survival and maintaining genome integrity. DSBs are repaired in a stepwise manner by homologous recombination. Here, we focused on the early steps of DSB repair, including DSB recognition, which is still only poorly understood. In prokaryotes, this process has been proposed to involve the RecN protein, a member of the structural maintenance of chromosome (SMC) protein family, which include key eukaryotic and prokaryotic proteins such as cohesin, condensin, and Rad50. An extensive high- and low-resolution structural analysis of Deinococcus radiodurans RecN using a combination of protein crystallography and small-angle X-ray scattering enabled us to assemble a quasi-atomic model of the entire RecN protein, representing the complete structure of a SMC-like protein. These results, together with a thorough biochemical and mutational study of RecN, allow us to propose a model for the role of RecN in DSB repair.
Organizational Affiliation:
Structural Biology Group, European Synchrotron Radiation Facility, BP 220, 38043 Grenoble Cedex 9, France.