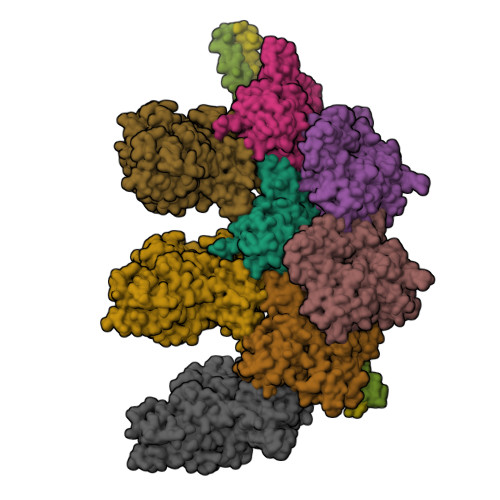
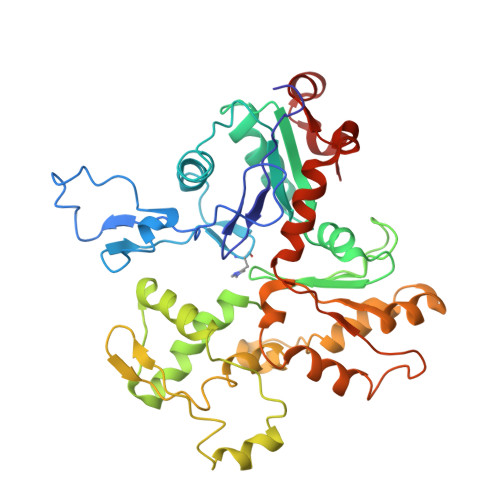
Structure of the Rigor Actin-Tropomyosin-Myosin Complex.
Behrmann, E., Muller, M., Penczek, P.A., Mannherz, H.G., Manstein, D.J., Raunser, S.(2012) Cell 150: 327
- PubMed: 22817895
- DOI: https://doi.org/10.1016/j.cell.2012.05.037
- Primary Citation of Related Structures:
4A7F, 4A7H, 4A7L, 4A7N - PubMed Abstract:
Regulation of myosin and filamentous actin interaction by tropomyosin is a central feature of contractile events in muscle and nonmuscle cells. However, little is known about molecular interactions within the complex and the trajectory of tropomyosin movement between its "open" and "closed" positions on the actin filament. Here, we report the 8 Å resolution structure of the rigor (nucleotide-free) actin-tropomyosin-myosin complex determined by cryo-electron microscopy. The pseudoatomic model of the complex, obtained from fitting crystal structures into the map, defines the large interface involving two adjacent actin monomers and one tropomyosin pseudorepeat per myosin contact. Severe forms of hereditary myopathies are linked to mutations that critically perturb this interface. Myosin binding results in a 23 Å shift of tropomyosin along actin. Complex domain motions occur in myosin, but not in actin. Based on our results, we propose a structural model for the tropomyosin-dependent modulation of myosin binding to actin.
Organizational Affiliation:
Department of Physical Biochemistry, Max Planck Institute of Molecular Physiology, 44227 Dortmund, Germany.