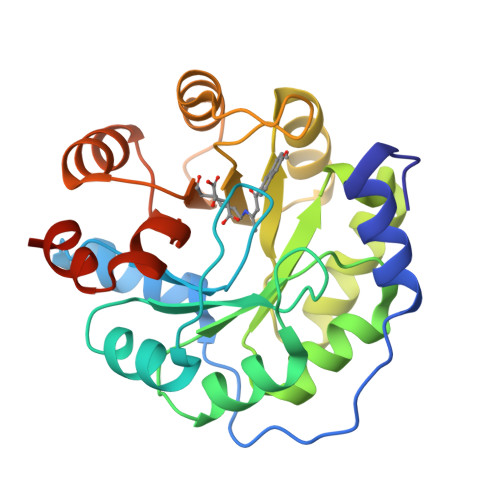
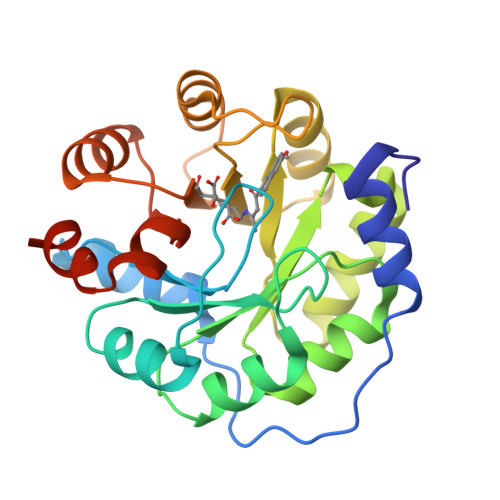
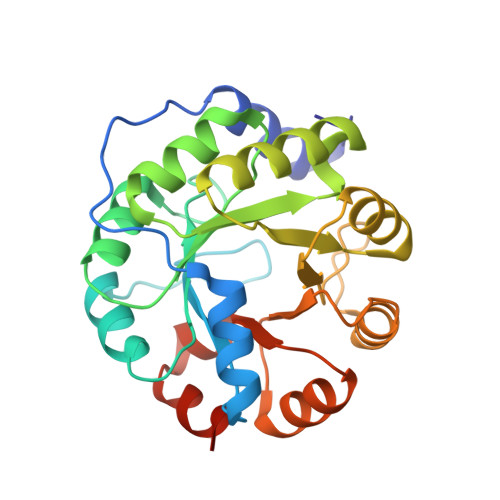
Evolution of a designed retro-aldolase leads to complete active site remodeling.
Giger, L., Caner, S., Obexer, R., Kast, P., Baker, D., Ban, N., Hilvert, D.(2013) Nat Chem Biol 9: 494-498
- PubMed: 23748672
- DOI: https://doi.org/10.1038/nchembio.1276
- Primary Citation of Related Structures:
4A29, 4A2R, 4A2S - PubMed Abstract:
Evolutionary advances are often fueled by unanticipated innovation. Directed evolution of a computationally designed enzyme suggests that pronounced molecular changes can also drive the optimization of primitive protein active sites. The specific activity of an artificial retro-aldolase was boosted >4,400-fold by random mutagenesis and screening, affording catalytic efficiencies approaching those of natural enzymes. However, structural and mechanistic studies reveal that the engineered catalytic apparatus, consisting of a reactive lysine and an ordered water molecule, was unexpectedly abandoned in favor of a new lysine residue in a substrate-binding pocket created during the optimization process. Structures of the initial in silico design, a mechanistically promiscuous intermediate and one of the most evolved variants highlight the importance of loop mobility and supporting functional groups in the emergence of the new catalytic center. Such internal competition between alternative reactive sites may have characterized the early evolution of many natural enzymes.
Organizational Affiliation:
Laboratory of Organic Chemistry, ETH Zurich, Zurich, Switzerland.