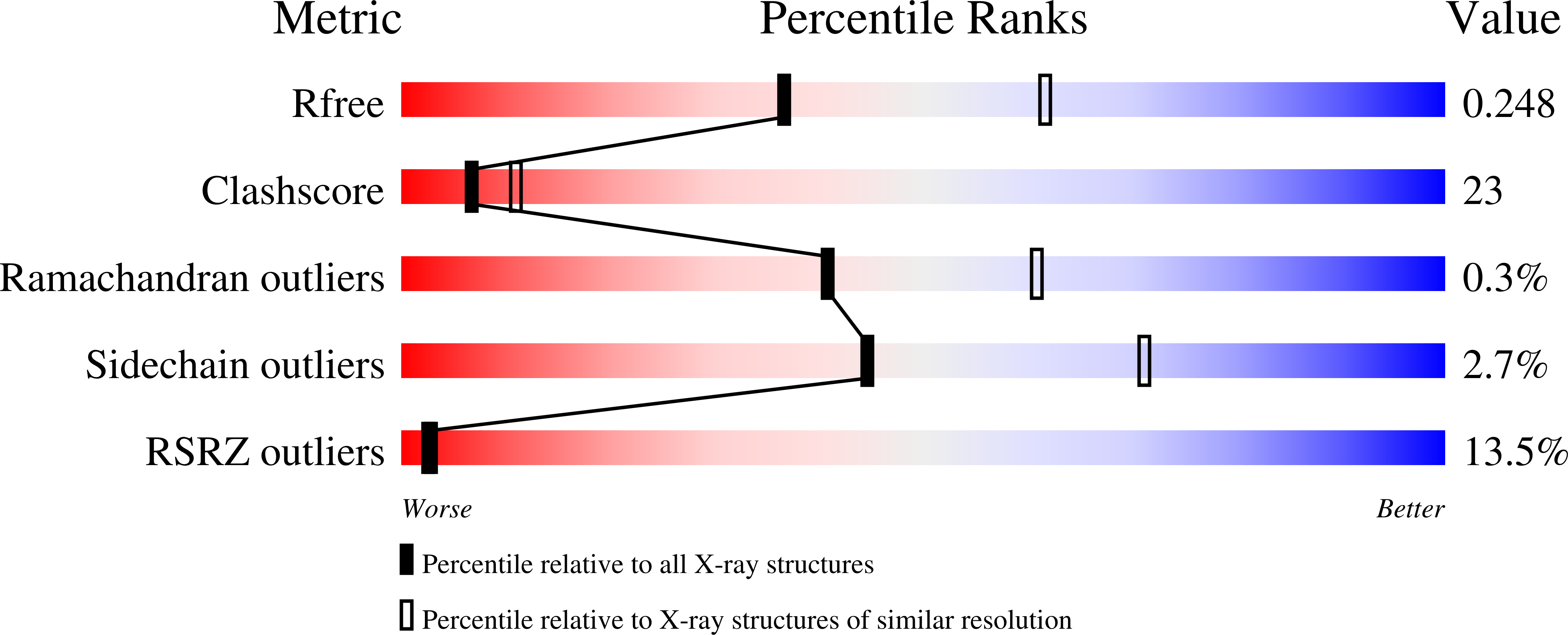
Architecture of a Dodecameric Bacterial Replicative Helicase.
Stelter, M., Gutsche, I., Kapp, U., Bazin, A., Bajic, G., Goret, G., Jamin, M., Timmins, J., Terradot, L.(2012) Structure 20: 554
- PubMed: 22405014
- DOI: https://doi.org/10.1016/j.str.2012.01.020
- Primary Citation of Related Structures:
4A1F - PubMed Abstract:
Hexameric DnaB helicases are often loaded at DNA replication forks by interacting with the initiator protein DnaA and/or a helicase loader (DnaC in Escherichia coli). These loaders are not universally required, and DnaB from Helicobacter pylori was found to bypass DnaC when expressed in E. coli cells. The crystal structure of Helicobacter pylori DnaB C-terminal domain (HpDnaB-CTD) reveals a large two-helix insertion (named HPI) in the ATPase domain that protrudes away from the RecA fold. Biophysical characterization and electron microscopy (EM) analysis of the full-length protein show that HpDnaB forms head-to-head double hexamers remarkably similar to helicases found in some eukaryotes, archaea, and viruses. The docking of the HpDnaB-CTD structure into EM reconstruction of HpDnaB provides a model that shows how hexamerization of the CTD is facilitated by HPI-HPI interactions. The HpDnaB double-hexamer architecture supports an alternative strategy to load bacterial helicases onto forks in the absence of helicase loaders.
Organizational Affiliation:
Structural Biology Group, European Synchrotron Radiation Facility, BP 220 38043 Grenoble Cedex 9, France.