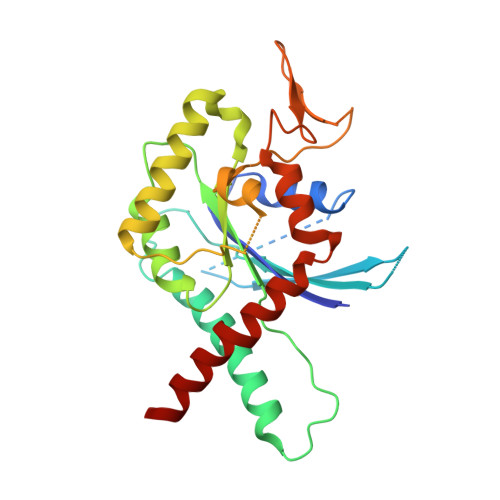
Structural and biochemical properties of Sept7, a unique septin required for filament formation.
Zent, E., Vetter, I., Wittinghofer, A.(2011) Biol Chem 392: 791-797
- PubMed: 21824007
- DOI: https://doi.org/10.1515/BC.2011.082
- Primary Citation of Related Structures:
3T5D - PubMed Abstract:
Septins constitute a family of conserved guanine nucleotide binding proteins found in a wide range of organisms from fungi to mammals. Members of the family share a canonical G-domain with N- and C-terminal extensions. G-domains assemble into hetero-oligomeric complexes which form non-polarised filaments or rings. Linear filaments are formed between the G-domains using either the guanine nucleotide binding site (G interface) or N- and C-terminal extensions (NC interface). Sept7 is a unique among the 13 human septins in that it occupies the ends of hexameric building blocks which assemble into non-polarised filaments. To gain insight into its particular properties we performed structural and biochemical studies on Sept7. We solved the crystal structure of a Sept7 dimer in the GDP-bound state. The structure and biochemistry of Sept7 provide new insights into the dynamics of the G interface and outline the differences in the properties of Sept7 compared to the members of group 2 septins.
- Structural Biology Group, Max Planck Institute of Molecular Physiology, Dortmund, Germany.
Organizational Affiliation: