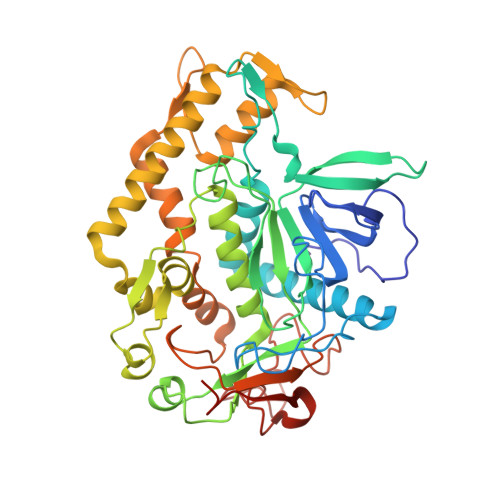
Peptide inhibitors of botulinum neurotoxin serotype A: design, inhibition, cocrystal structures, structure-activity relationship and pharmacophore modeling.
Kumar, G., Kumaran, D., Ahmed, S.A., Swaminathan, S.(2012) Acta Crystallogr D Biol Crystallogr 68: 511-520
- PubMed: 22525749
- DOI: https://doi.org/10.1107/S0907444912003551
- Primary Citation of Related Structures:
3QW5, 3QW6, 3QW7, 3QW8 - PubMed Abstract:
Clostridium botulinum neurotoxins are classified as Category A bioterrorism agents by the Centers for Disease Control and Prevention (CDC). The seven serotypes (A-G) of the botulinum neurotoxin, the causative agent of the disease botulism, block neurotransmitter release by specifically cleaving one of the three SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins and induce flaccid paralysis. Using a structure-based drug-design approach, a number of peptide inhibitors were designed and their inhibitory activity against botulinum serotype A (BoNT/A) protease was determined. The most potent peptide, RRGF, inhibited BoNT/A protease with an IC(50) of 0.9 µM and a K(i) of 358 nM. High-resolution crystal structures of various peptide inhibitors in complex with the BoNT/A protease domain were also determined. Based on the inhibitory activities and the atomic interactions deduced from the cocrystal structures, the structure-activity relationship was analyzed and a pharmacophore model was developed. Unlike the currently available models, this pharmacophore model is based on a number of enzyme-inhibitor peptide cocrystal structures and improved the existing models significantly, incorporating new features.
- Biology Department, Brookhaven National Laboratory, Upton, NY 11973, USA.
Organizational Affiliation: