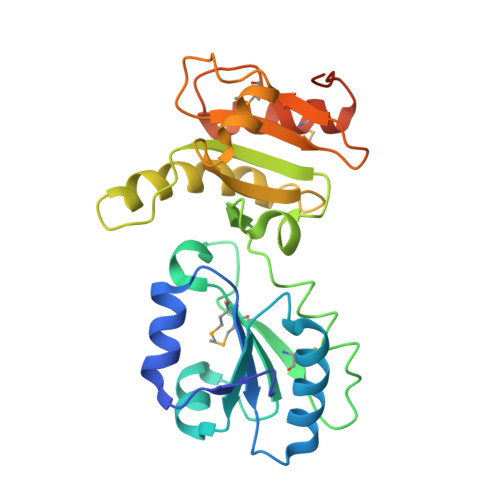
Structure of the Noncatalytic Domains and Global Fold of the Protein Disulfide Isomerase ERp72.
Kozlov, G., Maattanen, P., Schrag, J.D., Hura, G.L., Gabrielli, L., Cygler, M., Thomas, D.Y., Gehring, K.(2009) Structure 17: 651-659
- PubMed: 19446521
- DOI: https://doi.org/10.1016/j.str.2009.02.016
- Primary Citation of Related Structures:
3EC3 - PubMed Abstract:
Protein disulfide isomerases are a family of proteins that catalyze the oxidation and isomerization of disulfide bonds in newly synthesized proteins in the endoplasmic reticulum. The family includes general enzymes such as PDI that recognize unfolded proteins, and others that are selective for specific classes of proteins. Here, we report the X-ray crystal structure of central non-catalytic domains of a specific isomerase, ERp72 (also called CaBP2 and protein disulfide-isomerase A4) from Rattus norvegicus. The structure reveals strong similarity to ERp57, a PDI-family member that interacts with the lectin-like chaperones calnexin and calreticulin but, unexpectedly, ERp72 does not interact with calnexin as shown by isothermal titration calorimetry and nuclear magnetic resonance (NMR) spectroscopy. Small-angle X-ray scattering (SAXS) of ERp72 was used to develop models of the full-length protein using both rigid body refinement and ab initio simulated annealing of dummy atoms. The two methods show excellent agreement and define the relative positions of the five thioredoxin-like domains of ERp72 and potential substrate or chaperone binding sites.
- Department of Biochemistry, McGill University, 3655 Promenade Sir William Osler, Montréal, Québec, Canada.
Organizational Affiliation: