Crystal Structure of the Trp RNA-Binding Attenuation Protein (Trap) from Bacillus Licheniformis.
Shevtsov, M.B., Chen, Y., Gollnick, P., Antson, A.A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| TRYPTOPHAN OPERON RNA-BINDING ATTENUATION PROTEIN (TRAP) | 78 | Bacillus licheniformis | Mutation(s): 0 | 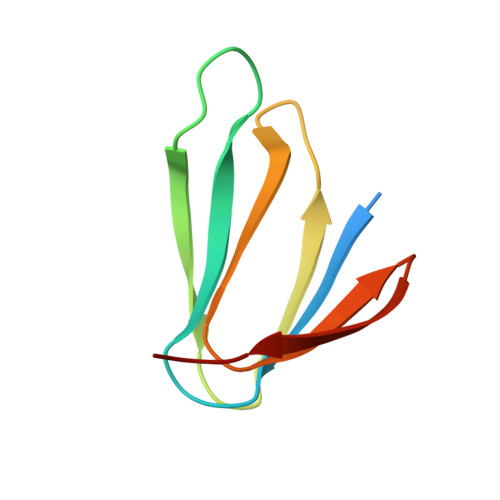 | |
UniProt | |||||
Find proteins for A5A665 (Bacillus licheniformis (strain ATCC 14580 / DSM 13 / JCM 2505 / CCUG 7422 / NBRC 12200 / NCIMB 9375 / NCTC 10341 / NRRL NRS-1264 / Gibson 46)) Explore A5A665 Go to UniProtKB: A5A665 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5A665 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| TRP Query on TRP | AA [auth E] BA [auth F] CA [auth G] DA [auth H] EA [auth I] | TRYPTOPHAN C11 H12 N2 O2 QIVBCDIJIAJPQS-VIFPVBQESA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 145.427 | α = 90 |
| b = 145.427 | β = 90 |
| c = 183.071 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |