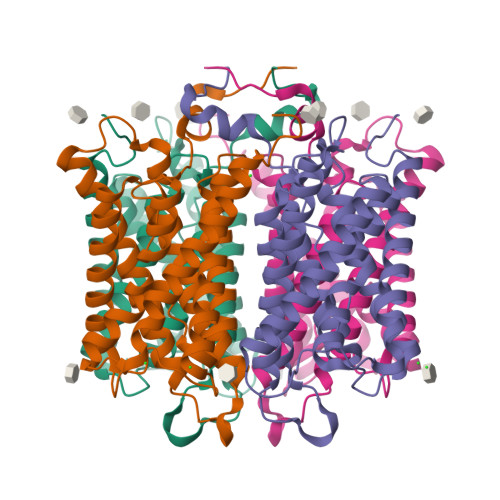
Subangstrom Resolution X-Ray Structure Details Aquaporin-Water Interactions
Kosinska-Eriksson, U., Fischer, G., Friemann, R., Enkavi, G., Tajkhorshid, E., Neutze, R.(2013) Science 340: 1346
- PubMed: 23766328
- DOI: https://doi.org/10.1126/science.1234306
- Primary Citation of Related Structures:
3ZOJ - PubMed Abstract:
Aquaporins are membrane channels that facilitate the flow of water across biological membranes. Two conserved regions are central for selective function: the dual asparagine-proline-alanine (NPA) aquaporin signature motif and the aromatic and arginine selectivity filter (SF). Here, we present the crystal structure of a yeast aquaporin at 0.88 angstrom resolution. We visualize the H-bond donor interactions of the NPA motif's asparagine residues to passing water molecules; observe a polarized water-water H-bond configuration within the channel; assign the tautomeric states of the SF histidine and arginine residues; and observe four SF water positions too closely spaced to be simultaneously occupied. Strongly correlated movements break the connectivity of SF waters to other water molecules within the channel and prevent proton transport via a Grotthuss mechanism.
Organizational Affiliation:
Department of Chemistry and Molecular Biology, University of Gothenburg, Box 462, S-40530 Göteborg, Sweden.