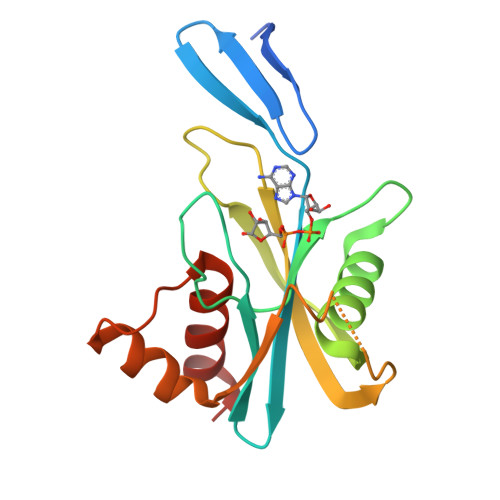
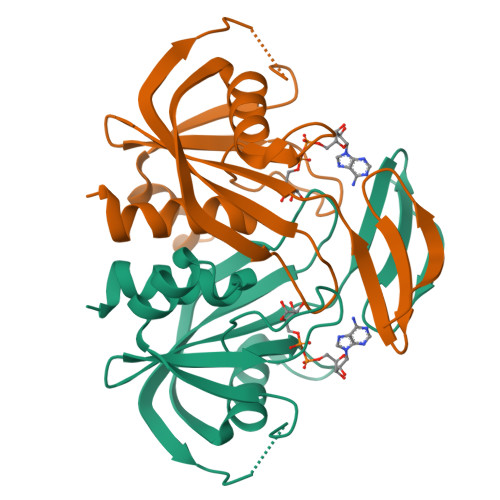
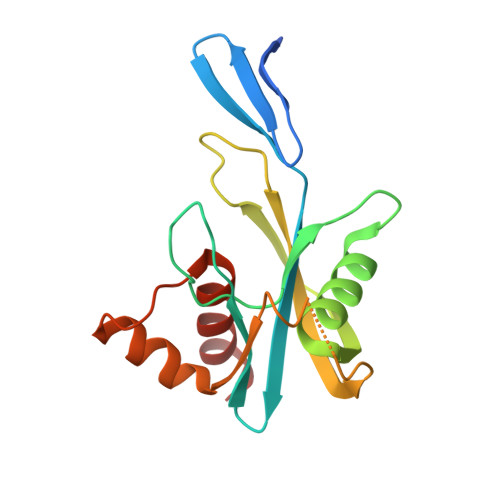
ADP-Ribose Pyrophosphatase Reaction in Crystalline State Conducted by Consecutive Binding of Two Manganese(II) Ions as Cofactors
Furuike, Y., Akita, Y., Miyahara, I., Kamiya, N.(2016) Biochemistry 55: 1801-1812
- PubMed: 26979298
- DOI: https://doi.org/10.1021/acs.biochem.5b00886
- Primary Citation of Related Structures:
3X0I, 3X0J, 3X0K, 3X0L, 3X0M, 3X0N, 3X0O, 3X0P, 3X0Q, 3X0R, 3X0S - PubMed Abstract:
Adenosine diphosphate ribose pyrophosphatase (ADPRase), a member of the Nudix family proteins, catalyzes the metal-induced and concerted general acid-base hydrolysis of ADP ribose (ADPR) into AMP and ribose-5'-phosphate (R5P). The ADPR-hydrolysis reaction of ADPRase from Thermus thermophilus HB8 (TtADPRase) requires divalent metal cations such as Mn(2+), Zn(2+), or Mg(2+) as cofactors. Here, we report the reaction pathway observed in the catalytic center of TtADPRase, based on cryo-trapping X-ray crystallography at atomic resolutions around 1.0 Å using Mn(2+) as the reaction trigger, which was soaked into TtADPRase-ADPR binary complex crystals. Integrating 11 structures along the reaction timeline, five reaction states of TtADPRase were assigned, which were ADPRase alone (E), the ADPRase-ADPR binary complex (ES), two ADPRase-ADPR-Mn(2+) reaction intermediates (ESM, ESMM), and the postreaction state (E'). Two Mn(2+) ions were inserted consecutively into the catalytic center of the ES-state and ligated by Glu86 and Glu82, which are highly conserved among the Nudix family, in the ESM- and ESMM-states. The ADPR-hydrolysis reaction was characterized by electrostatic, proximity, and orientation effects, and by preferential binding for the transition state. A new reaction mechanism is proposed, which differs from previous ones suggested from structure analyses with nonhydrolyzable substrate analogues or point-mutated ADPRases.
Organizational Affiliation:
Graduate School of Science, Osaka City University , 3-3-138 Sugimoto, Sumiyoshi, Osaka 558-8585, Japan.