An intramolecular bond at cluster of differentiation 81 ectodomain is important for hepatitis C virus entry.
Yang, W., Zhang, M., Chi, X., Liu, X., Qin, B., Cui, S.(2015) FASEB J 29: 4214-4226
- PubMed: 26116703
- DOI: https://doi.org/10.1096/fj.15-272880
- Primary Citation of Related Structures:
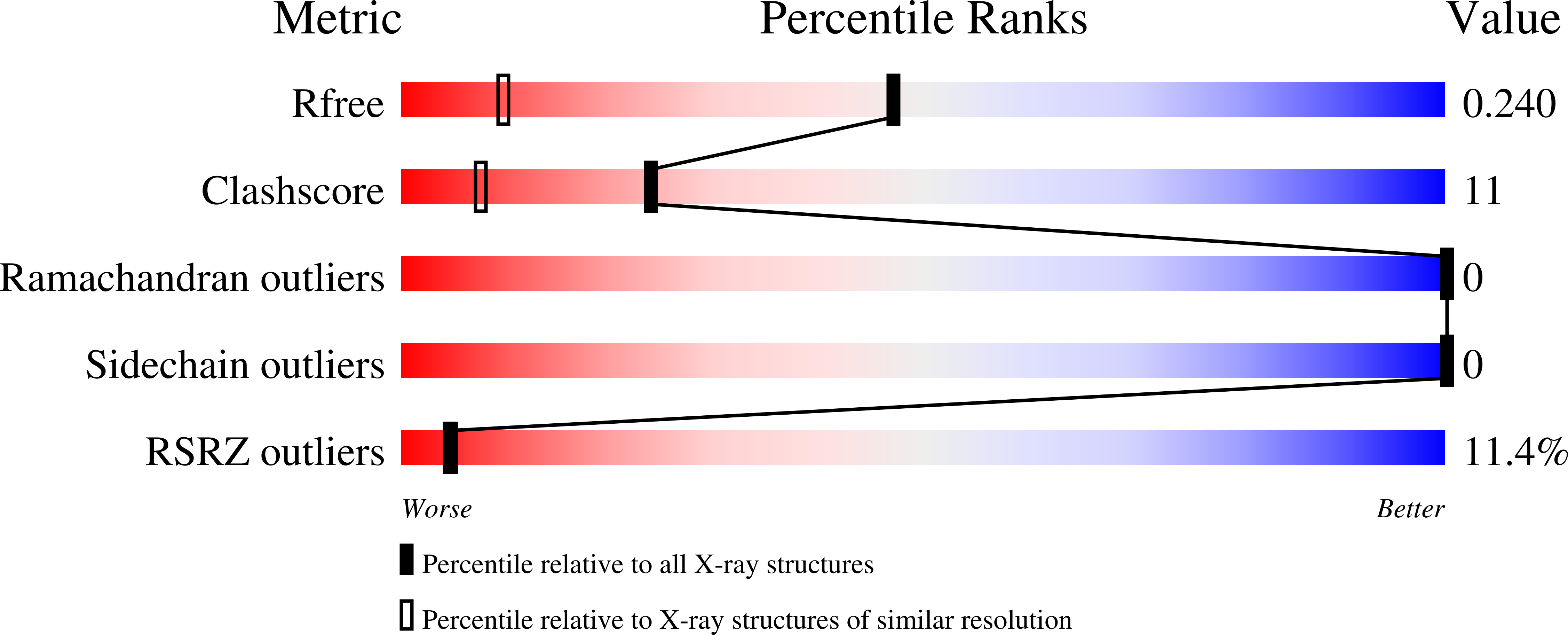
3X0E, 3X0F, 3X0G - PubMed Abstract:
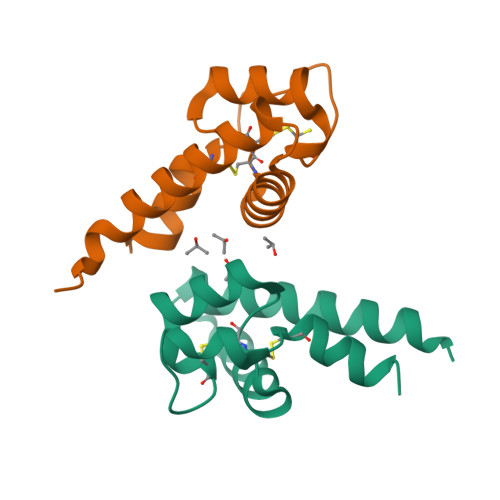
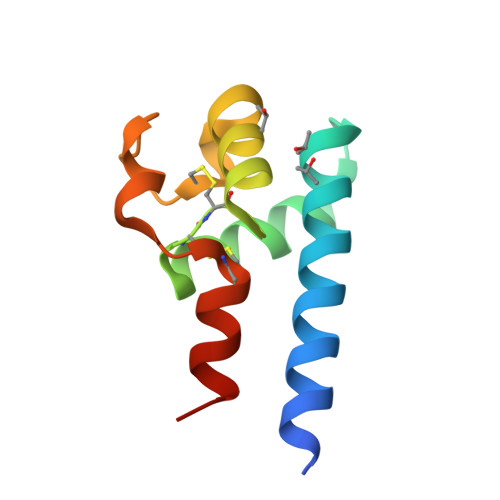
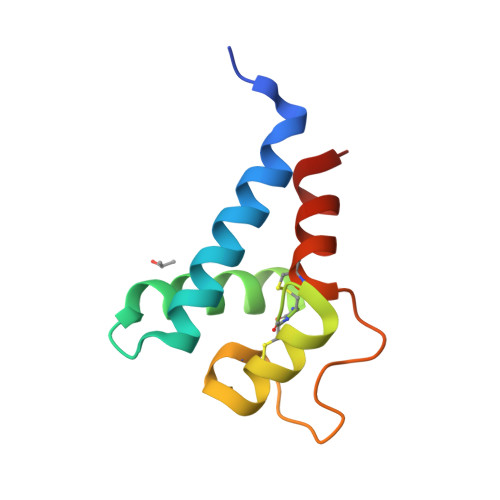
Hepatitis C virus (HCV) infection is one of the leading causes of chronic liver diseases; however, HCV vaccine remains unavailable to date. One main obstacle is the lack of an efficient small animal model. Cluster of differentiation 81 (CD81) is an essential entry coreceptor for HCV species specificity to humans, though the underlying mechanisms are yet to be fully elucidated. We performed structural, biophysical, and virologic studies on HCV nonpermissive CD81s from mice and African green monkeys [mouse cluster of differentiation 81 (mCD81) and African green monkey cluster of differentiation 81 (agmCD81)] and compared with human cluster of differentiation 81 (hCD81). We discovered an intramolecular hydrogen bond (Gln188-Nε2-H: Glu196-Oε2, 2 Å, 124°) within the large extracellular loop (LEL) of mCD81 and a salt bridge (Lys188-Nζ: Asp196-Oδ2, 2.4 Å) within agmCD81-LEL between residues 188 and 196. This structural feature is missing in hCD81. We demonstrated that the introduction of a single 188-196 bond to hCD81 impaired its binding affinity to HCV envelope glycoprotein 2 (HCV E2) and significantly decreased HCV pseudoviral particle (HCVpp) entry efficiency (4.92- to 8.42-fold) and cell culture-grown HCV (HCVcc) infectivity (4.55-fold), despite the availability of Phe186. For HCV nonpermissive CD81s, the introduction of Phe186 by Leu186F substitution alone was insufficient to confer HCV permissiveness. The disruption of the original 188-196 bond and Leu186F substitution were both required for potent binding to HCV E2 HCVpp entry efficiency and HCVcc infectivity. Our structural and biophysical analyses suggest that the intramolecular 188-196 bond restricts the intrinsic conformational dynamics of D-helix of CD81-LEL, which is essential for HCV entry, thus impairs HCV permissiveness. Our findings reveal a novel molecular determinant for HCV entry in addition to the well-characterized Phe186 and provide further guideline for selecting an HCV small animal model.
Organizational Affiliation:
Ministry of Health Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China wyang@ipb.pumc.edu.cn cui.sheng@ipb.pumc.edu.cn.