Discovery, Primary, and Crystal Structures and Capacitation-related Properties of a Prostate-derived Heparin-binding Protein WGA16 from Boar Sperm
Garenaux, E., Kanagawa, M., Tsuchiyama, T., Hori, K., Kanazawa, T., Goshima, A., Chiba, M., Yasue, H., Ikeda, A., Yamaguchi, Y., Sato, C., Kitajima, K.(2015) J Biological Chem 290: 5484-5501
- PubMed: 25568322
- DOI: https://doi.org/10.1074/jbc.M114.635268
- Primary Citation of Related Structures:
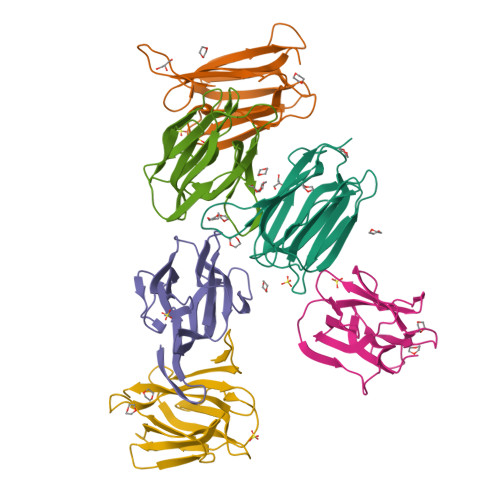
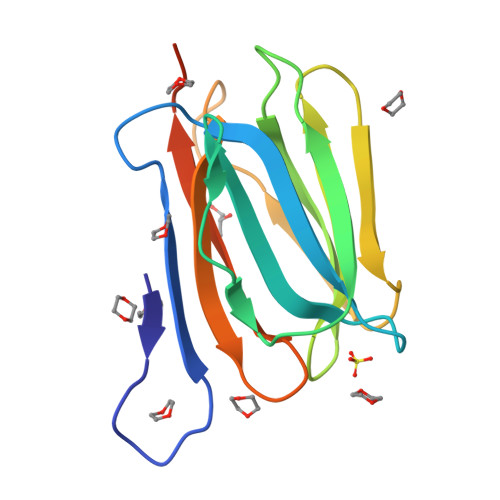
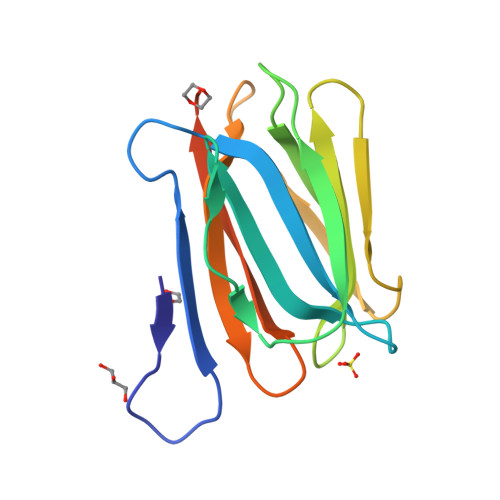
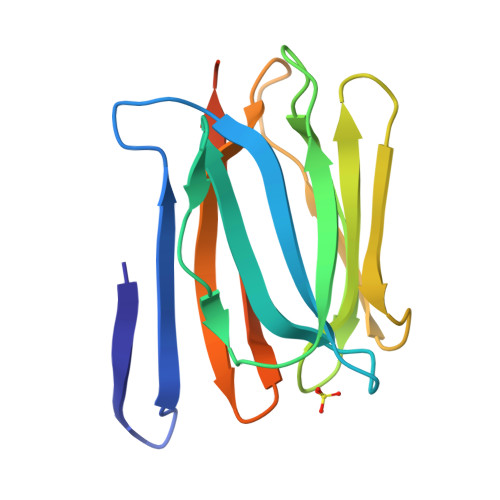
3WOB, 3WOC - PubMed Abstract:
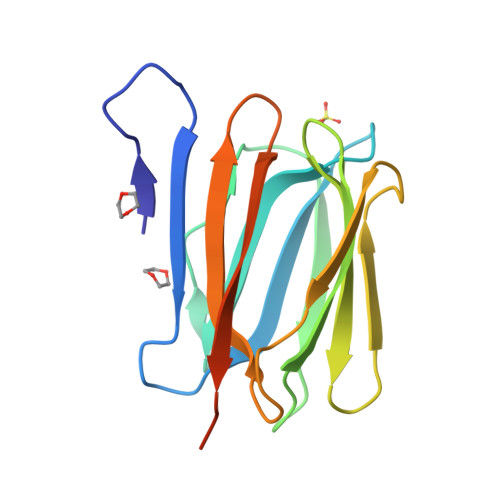
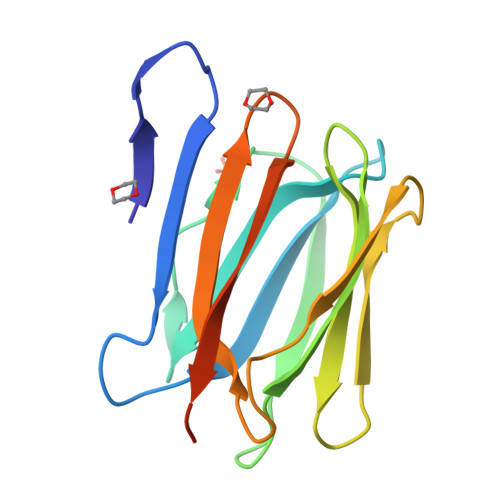
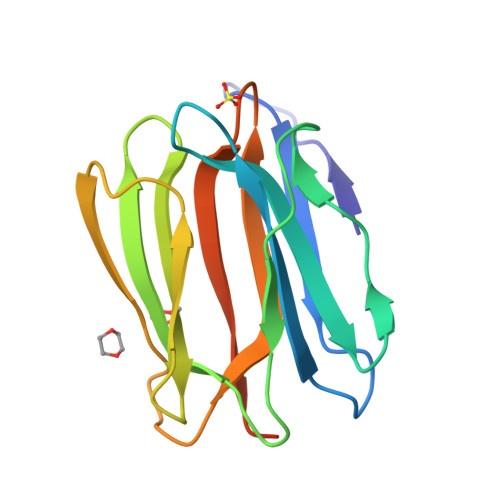
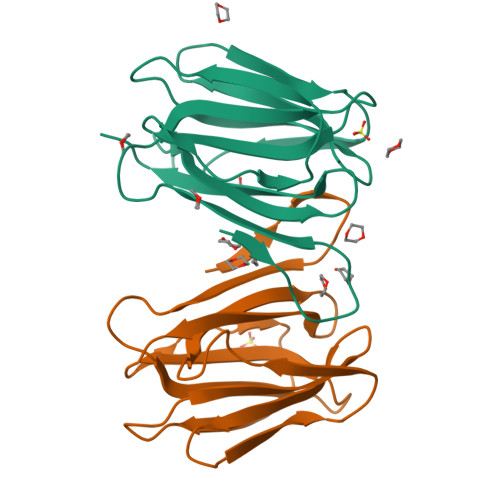
Mammalian sperm acquire fertility through a functional maturation process called capacitation, where sperm membrane molecules are drastically remodeled. In this study, we found that a wheat germ agglutinin (WGA)-reactive protein on lipid rafts, named WGA16, is removed from the sperm surface on capacitation. WGA16 is a prostate-derived seminal plasma protein that has never been reported and is deposited on the sperm surface in the male reproductive tract. Based on protein and cDNA sequences for purified WGA16, it is a homologue of human zymogen granule protein 16 (ZG16) belonging to the Jacalin-related lectin (JRL) family in crystal and primary structures. A glycan array shows that WGA16 binds heparin through a basic patch containing Lys-53/Lys-73 residues but not the conventional lectin domain of the JRL family. WGA16 is glycosylated, contrary to other ZG16 members, and comparative mass spectrometry clearly shows its unique N-glycosylation profile among seminal plasma proteins. It has exposed GlcNAc and GalNAc residues without additional Gal residues. The GlcNAc/GalNAc residues can work as binding ligands for a sperm surface galactosyltransferase, which actually galactosylates WGA16 in situ in the presence of UDP-Gal. Interestingly, surface removal of WGA16 is experimentally induced by either UDP-Gal or heparin. In the crystal structure, N-glycosylated sites and a potential heparin-binding site face opposite sides. This geography of two functional sites suggest that WGA16 is deposited on the sperm surface through interaction between its N-glycans and the surface galactosyltransferase, whereas its heparin-binding domain may be involved in binding to sulfated glycosaminoglycans in the female tract, enabling removal of WGA16 from the sperm surface.
Organizational Affiliation:
From the Bioscience and Biotechnology Center, Nagoya University, Nagoya 464-8601, Japan.