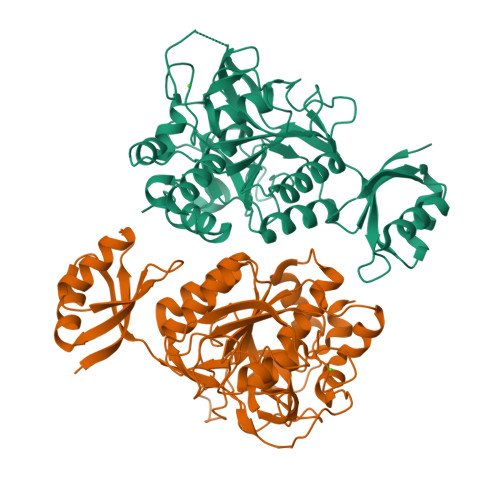
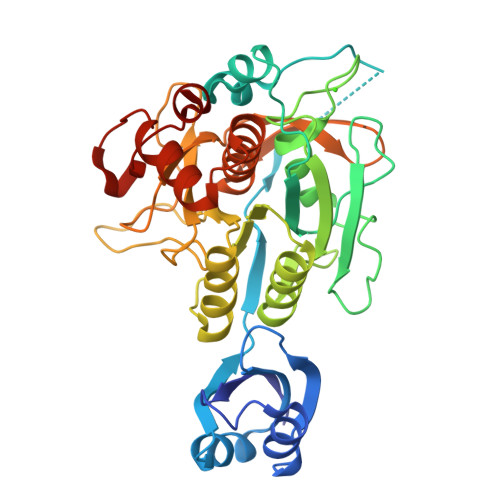
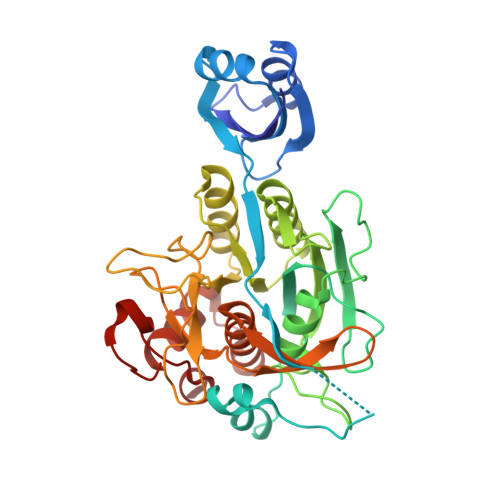
Formation of the High-Affinity Calcium Binding Site in Pro-subtilisin E with the Insertion Sequence IS1 of Pro-Tk-subtilisin
Uehara, R., Angkawidjaja, C., Koga, Y., Kanaya, S.(2013) Biochemistry 52: 9080-9088
- PubMed: 24279884
- DOI: https://doi.org/10.1021/bi401342k
- Primary Citation of Related Structures:
3WHI - PubMed Abstract:
Subtilisin E is activated from its inactive precursor Pro-subtilisin E by autoprocessing and degradation of the propeptide. Subtilisin E has two calcium binding sites, the high-affinity Ca1 site and the low-affinity Ca2 site. The Ca1 site is conserved in various subtilisin-like proteases and is important for stability. This site is not formed in Pro-subtilisin E, because the structural rearrangement of the N-terminal region of the subtilisin domain upon autoprocessing is necessary for the formation of this site. As a result, Pro-subtilisin E is not fully folded. In contrast, Pro-Tk-subtilisin from Thermococcus kodakarensis is fully folded, because it does not require the structural rearrangement upon autoprocessing for the formation of the Ca1 site due to the presence of the insertion sequence IS1 between the propeptide and subtilisin domains. To examine whether the Ca1 site is formed in Pro-subtilisin E by inserting IS1 between the propeptide and subtilisin domains, the Pro-subtilisin E mutant with this insertion, IS1-Pro-subtilisin E, and its active site mutants, IS1-Pro-S221A and IS1-Pro-S221C, were constructed and characterized. The crystal structure of IS1-Pro-S221A revealed that this protein is fully folded and the Ca1 site is formed. In this structure, IS1 serves as a linker that brings the N-terminus of the subtilisin domain near the Ca1 site. IS1-Pro-S221A in a calcium-bound form was more stable than that in a calcium-free form by 13.1 °C. IS1-Pro-S221C was more rapidly autoprocessed than Pro-S221C. These results suggest that IS1 facilitates the formation of the Ca1 site and the complete folding of Pro-subtilisin E and thereby accelerates its autoprocessing.
Organizational Affiliation:
Department of Material and Life Science, Graduate School of Engineering, Osaka University , 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan.