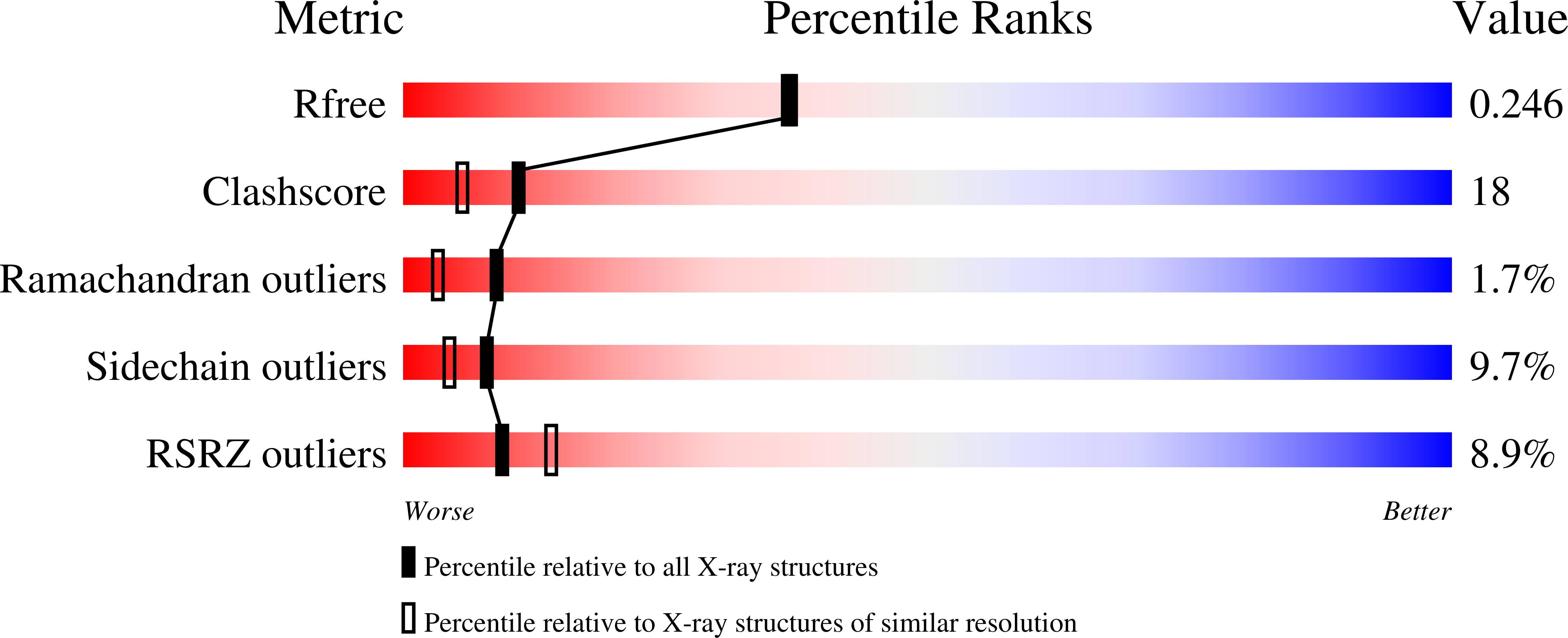
Crystal structure of the membrane-bound bifunctional transglycosylase PBP1b from Escherichia coli.
Sung, M.T., Lai, Y.T., Huang, C.Y., Chou, L.Y., Shih, H.W., Cheng, W.C., Wong, C.H., Ma, C.(2009) Proc Natl Acad Sci U S A 106: 8824-8829
- PubMed: 19458048
- DOI: https://doi.org/10.1073/pnas.0904030106
- Primary Citation of Related Structures:
3FWL, 3VMA - PubMed Abstract:
Drug-resistant bacteria have caused serious medical problems in recent years, and the need for new antibacterial agents is undisputed. Transglycosylase, a multidomain membrane protein essential for cell wall synthesis, is an excellent target for the development of new antibiotics. Here, we determined the X-ray crystal structure of the bifunctional transglycosylase penicillin-binding protein 1b (PBP1b) from Escherichia coli in complex with its inhibitor moenomycin to 2.16-A resolution. In addition to the transglycosylase and transpeptidase domains, our structure provides a complete visualization of this important antibacterial target, and reveals a domain for protein-protein interaction and a transmembrane helix domain essential for substrate binding, enzymatic activity, and membrane orientation.
Organizational Affiliation:
Genomics Research Center, Academia Sinica, 128 Academia Road, Section 2, Taipei 115, Taiwan.