Recognition of heteropolysaccharide alginate by periplasmic solute-binding proteins of a bacterial ABC transporter
Nishitani, Y., Maruyama, Y., Itoh, T., Mikami, B., Hashimoto, W., Murata, K.(2012) Biochemistry 51: 3622-3633
- PubMed: 22486720
- DOI: https://doi.org/10.1021/bi300194f
- Primary Citation of Related Structures:
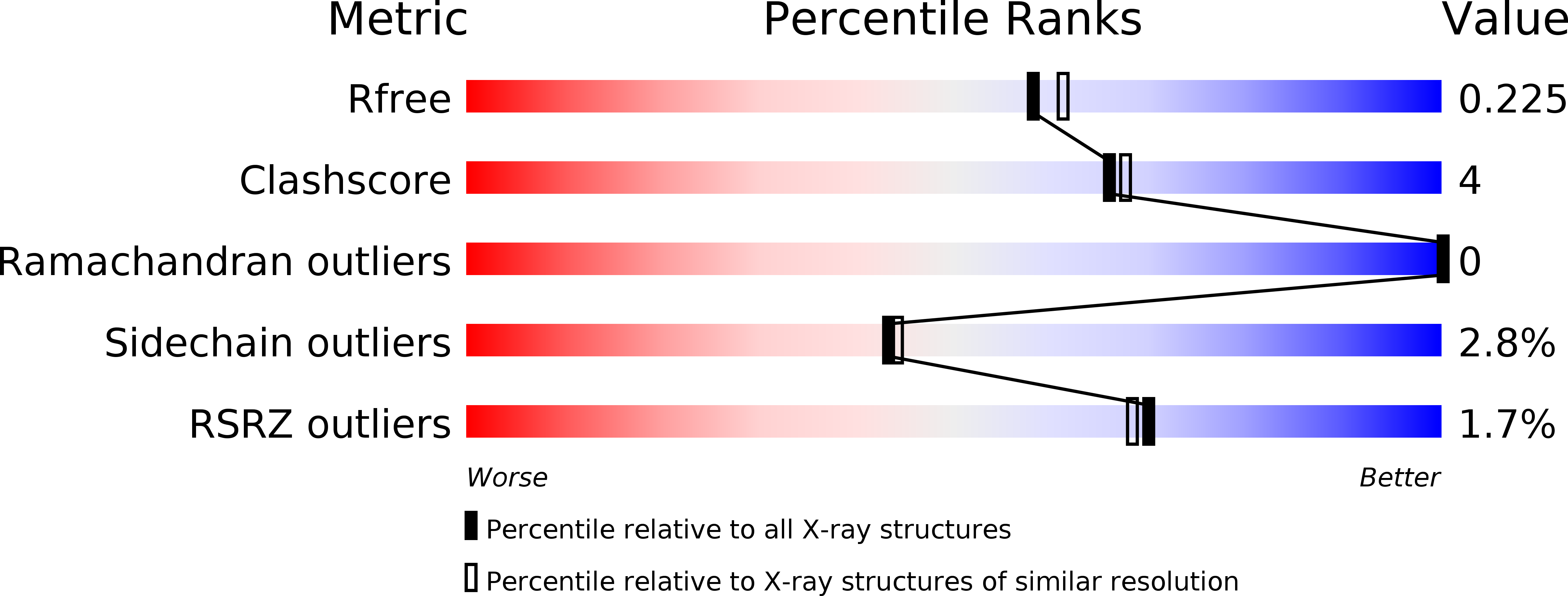
3VLU, 3VLV, 3VLW - PubMed Abstract:
Alginate is a heteropolysaccharide that consists of β-D-mannuronate (M) and α-L-guluronate (G). The Gram-negative bacterium Sphingomonas sp. A1 directly incorporates alginate into the cytoplasm through the periplasmic solute-binding protein (AlgQ1 and AlgQ2)-dependent ABC transporter (AlgM1-AlgM2/AlgS-AlgS). Two binding proteins with at least four subsites strongly recognize the nonreducing terminal residue of alginate at subsite 1. Here, we show the broad substrate preference of strain A1 solute-binding proteins for M and G present in alginate and demonstrate the structural determinants in binding proteins for heteropolysaccharide recognition through X-ray crystallography of four AlgQ1 structures in complex with saturated and unsaturated alginate oligosaccharides. Alginates with different M/G ratios were assimilated by strain A1 cells and bound to AlgQ1 and AlgQ2. Crystal structures of oligosaccharide-bound forms revealed that in addition to interaction between AlgQ1 and unsaturated oligosaccharides, the binding protein binds through hydrogen bonds to the C4 hydroxyl group of the saturated nonreducing terminal residue at subsite 1. The M residue of saturated oligosaccharides is predominantly accommodated at subsite 1 because of the strict binding of Ser-273 to the carboxyl group of the residue. In unsaturated trisaccharide (ΔGGG or ΔMMM)-bound AlgQ1, the protein interacts appropriately with substrate hydroxyl groups at subsites 2 and 3 to accommodate M or G, while substrate carboxyl groups are strictly recognized by the specific residues Tyr-129 at subsite 2 and Lys-22 at subsite 3. Because of this substrate recognition mechanism, strain A1 solute-binding proteins can bind heteropolysaccharide alginate with different M/G ratios.
Organizational Affiliation:
Laboratory of Basic and Applied Molecular Biotechnology, Graduate School of Agriculture, Kyoto University, Uji, Kyoto 611-0011, Japan.