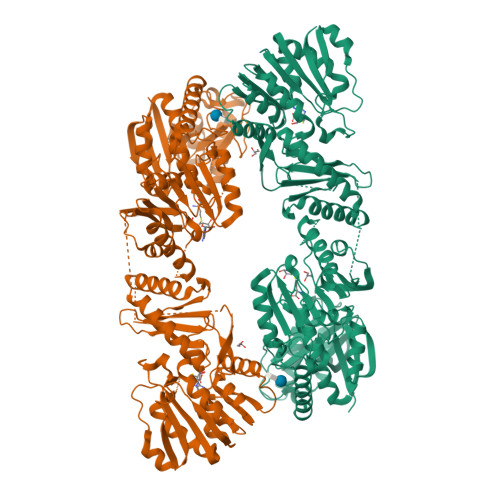
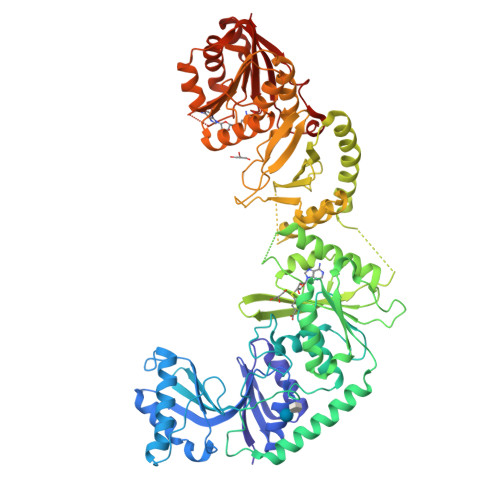
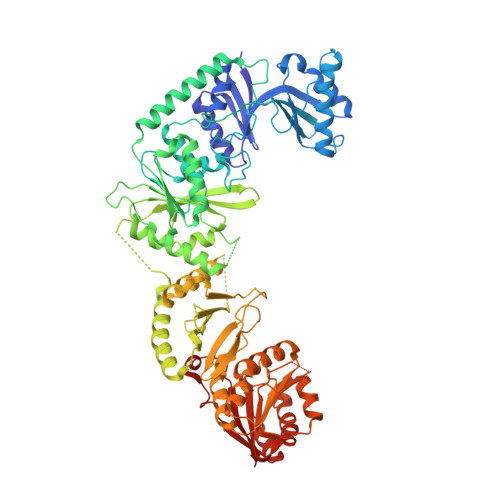
Structure of the bifunctional methyltransferase YcbY (RlmKL) that adds the m7G2069 and m2G2445 modifications in Escherichia coli 23S rRNA
Wang, K.T., Desmolaize, B., Nan, J., Zhang, X.W., Li, L.F., Douthwaite, S., Su, X.D.(2012) Nucleic Acids Res 40: 5138-5148
- PubMed: 22362734
- DOI: https://doi.org/10.1093/nar/gks160
- Primary Citation of Related Structures:
3V8V, 3V97 - PubMed Abstract:
The 23S rRNA nucleotide m(2)G2445 is highly conserved in bacteria, and in Escherichia coli this modification is added by the enzyme YcbY. With lengths of around 700 amino acids, YcbY orthologs are the largest rRNA methyltransferases identified in Gram-negative bacteria, and they appear to be fusions from two separate proteins found in Gram-positives. The crystal structures described here show that both the N- and C-terminal halves of E. coli YcbY have a methyltransferase active site and their folding patterns respectively resemble the Streptococcus mutans proteins Smu472 and Smu776. Mass spectrometric analyses of 23S rRNAs showed that the N-terminal region of YcbY and Smu472 are functionally equivalent and add the m(2)G2445 modification, while the C-terminal region of YcbY is responsible for the m(7)G2069 methylation on the opposite side of the same helix (H74). Smu776 does not target G2069, and this nucleotide remains unmodified in Gram-positive rRNAs. The E.coli YcbY enzyme is the first example of a methyltransferase catalyzing two mechanistically different types of RNA modification, and has been renamed as the Ribosomal large subunit methyltransferase, RlmKL. Our structural and functional data provide insights into how this bifunctional enzyme evolved.
Organizational Affiliation:
State Key Laboratory of Protein and Plant Gene Research, and Biodynamic Optical Imaging Center (BIOPIC), School of Life Sciences, Peking University, Beijing 100871, Republic of China.