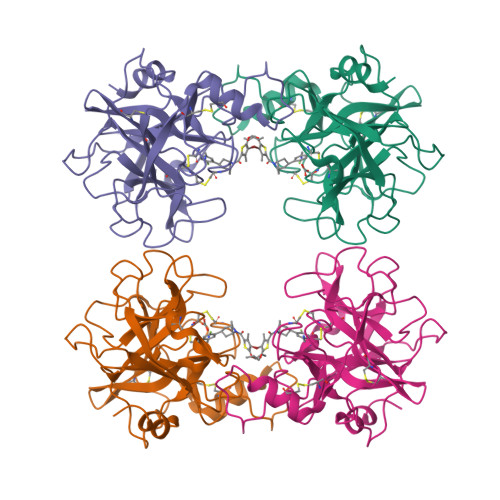
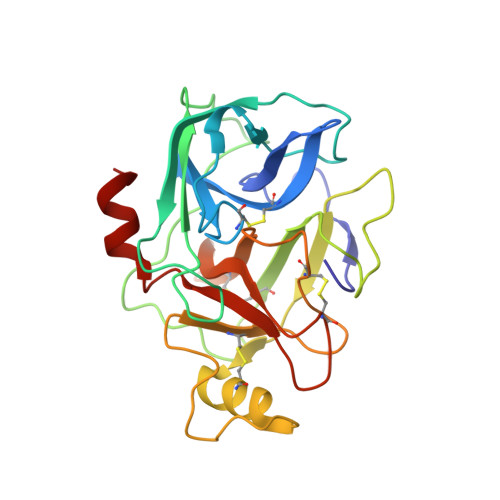
A beta-tryptase inhibitor with a tropanylamide scaffold to improve in vitro stability and to lower hERG channel binding affinity
Liang, G., Choi-Sledeski, Y.M., Shum, P., Chen, X., Poli, G.B., Kumar, V., Minnich, A., Wang, Q., Tsay, J., Sides, K., Kang, J., Zhang, Y.(2012) Bioorg Med Chem Lett 22: 1606-1610
- PubMed: 22264487
- DOI: https://doi.org/10.1016/j.bmcl.2011.12.127
- Primary Citation of Related Structures:
3V7T - PubMed Abstract:
Tropanylamide was investigated as a possible scaffold for β-tryptase inhibitors with a basic benzylamine P1 group and a substituted thiophene P4 group. Comparing to piperidinylamide, the tropanylamide scaffold is much more rigid, which presents less opportunity for the inhibitor to bind with off-target proteins, such as cytochrome P450, SSAO, and hERG potassium channel. The proposed binding mode was further confirmed by an in-house X-ray structure through co-crystallization.
Organizational Affiliation:
Molecular Innovative Therapeutics, Sanofi Pharmaceuticals, Bridgewater, NJ 08807, USA. guyan.liang@sanofi-aventis.com