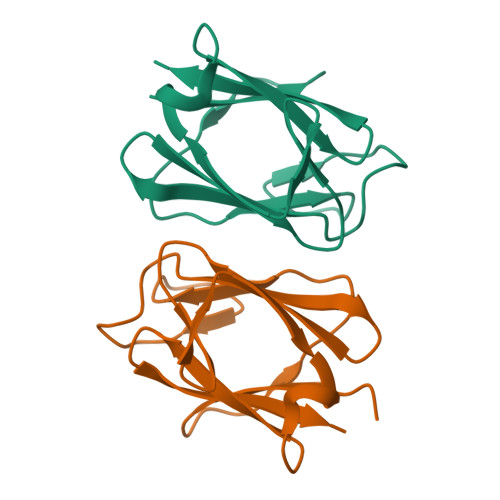
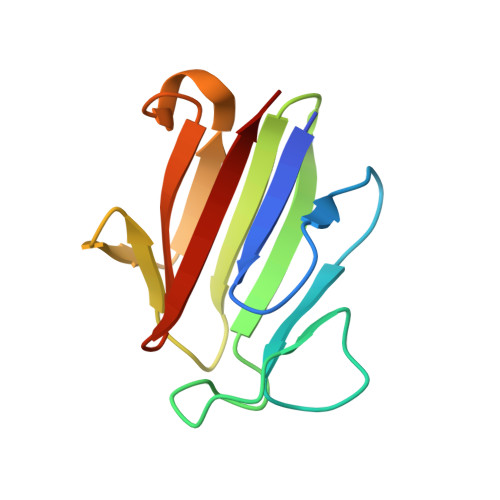
Dimerization, but not phosphothreonine binding, is conserved between the forkhead-associated domains of Drosophila MU2 and human MDC1
Luo, S., Ye, K.(2012) FEBS Lett 586: 344-349
- PubMed: 22273583
- DOI: https://doi.org/10.1016/j.febslet.2012.01.023
- Primary Citation of Related Structures:
3UV0 - PubMed Abstract:
Mutator 2 (MU2) in Drosophila melanogaster has been proposed to be the ortholog of human MDC1, a key mediator in DNA damage response. The forkhead-associated (FHA) domain of MDC1 is a dimerization module regulated by trans binding to phosphothreonine 4 from another molecule. Here we present the crystal structure of the MU2 FHA domain at 1.9Å resolution, revealing its evolutionarily conserved role in dimerization. As compared to the MDC1 FHA domain, the MU2 FHA domain dimerizes using a different and more stable interface and contains a degenerate phosphothreonine-binding pocket. Our results suggest that the MU2 dimerization is constitutive and lacks phosphorylation-mediated regulation.
Organizational Affiliation:
College of Biological Sciences, China Agricultural University, Beijing, China.