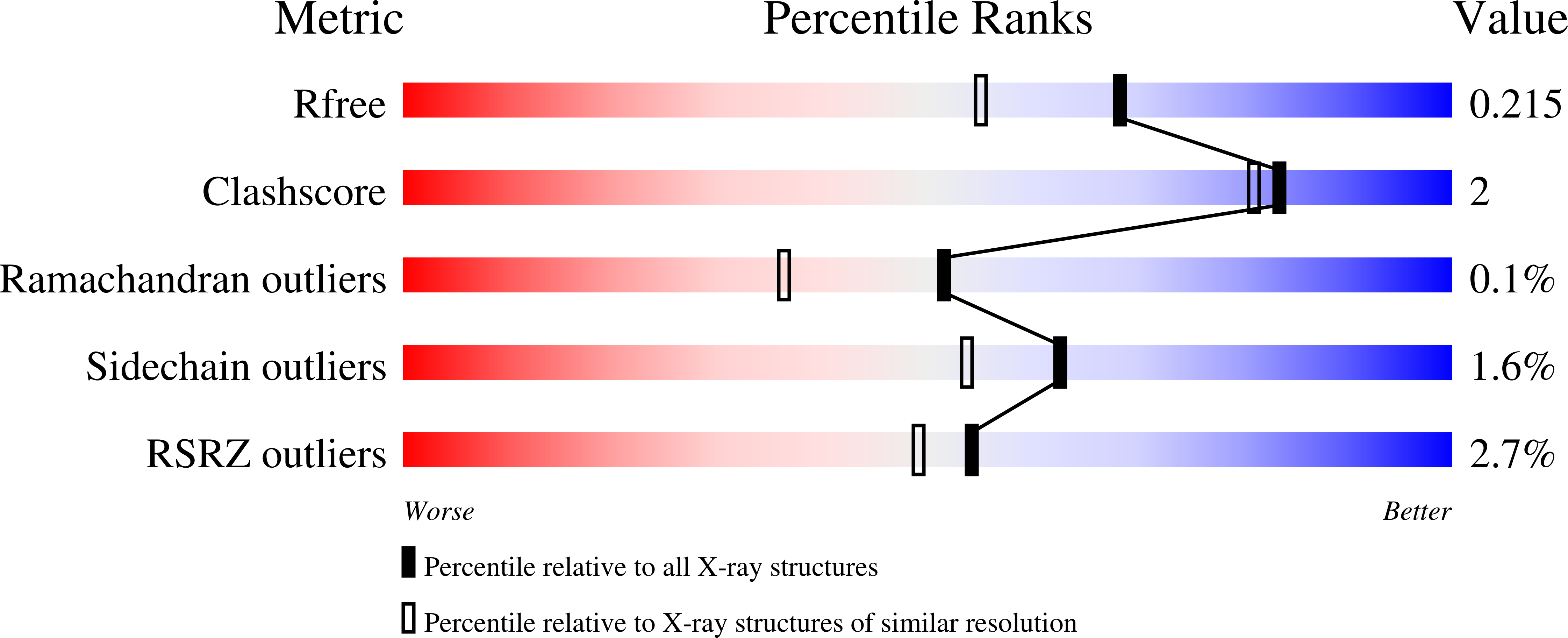
Structural and biochemical characterization of an atypical short-chain dehydrogenase/reductase reveals an unusual cofactor preference.
Buysschaert, G., Verstraete, K., Savvides, S.N., Vergauwen, B.(2013) FEBS J 280: 1358-1370
- PubMed: 23311896
- DOI: https://doi.org/10.1111/febs.12128
- Primary Citation of Related Structures:
3UCE, 3UCF - PubMed Abstract:
Short-chain dehydrogenases/reductases (SDRs) encompass a large and functionally diverse family of enzymes with representative members in all kingdoms of life. Despite the wealth of reactions catalyzed by SDRs, they operate through a well-conserved and efficient reaction mechanism centered in a conserved catalytic tetrad (Asn-Ser-Tyr-Lys) and the employment of an appropriate cofactor. In recent years, SDRs that lack the signature catalytic tetrad have been identified, thus adding a perplexing twist to SDR functionality. In the present study, we report the crystal structure of SDRvv, an atypical SDR from Vibrio vulnificus devoid of the catalytic tetrad, thereby defining the structural signature of this apparent SDR family outlier. Further structural analysis of SDRvv in complex with its putative cofactor NADPH, site-directed mutagenesis and binding studies via isothermal titration calorimetry, and further biochemical characterization have allowed us to dissect the cofactor preferences of SDRvv. The retained capacity to bind the NADPH cofactor, the conceivable existence of a proton relay and the conservation of the coordination distances between the key residues in the cofactor binding pocket define a first set of rules towards catalytic activity for SDRvv. The findings of the present study set the stage for deriving the identity of the natural substrate of SDRvv and add a new twist to the structure-function landscape for Rossmann-fold-dependent cofactor discrimination.
Organizational Affiliation:
Unit for Structural Biology, Laboratory for Protein Biochemistry and Biomolecular Engineering (L-ProBE), Ghent University, Ghent, Belgium.