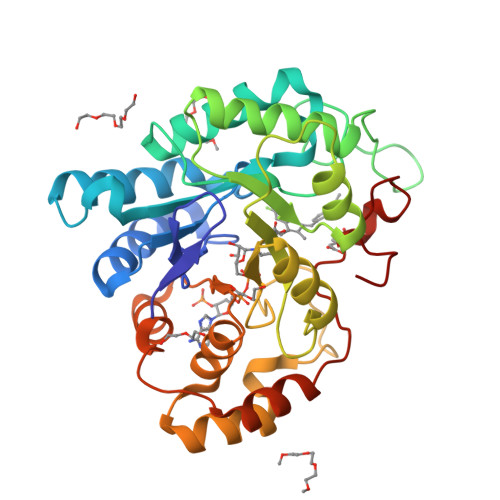
An old NSAID revisited: crystal structure of aldose reductase in complex with sulindac at 1.0 A supports a novel mechanism for its anticancer and antiproliferative effects.
Steuber, H.(2011) ChemMedChem 6: 2155-2157
- PubMed: 21997888
- DOI: https://doi.org/10.1002/cmdc.201100374
- Primary Citation of Related Structures:
3U2C
Organizational Affiliation:
Proteros Biostructures GmbH, Am Klopferspitz 19, 82152 Martinsried, Germany. steuber@proteros.com