Structure of Hydrogenase Maturation Protein HypF with Reaction Intermediates Shows Two Active Sites.
Petkun, S., Shi, R., Li, Y., Asinas, A., Munger, C., Zhang, L., Waclawek, M., Soboh, B., Sawers, R.G., Cygler, M.(2011) Structure 19: 1773-1783
- PubMed: 22153500
- DOI: https://doi.org/10.1016/j.str.2011.09.023
- Primary Citation of Related Structures:
3TSP, 3TSQ, 3TSU, 3TTC, 3TTD, 3TTF - PubMed Abstract:
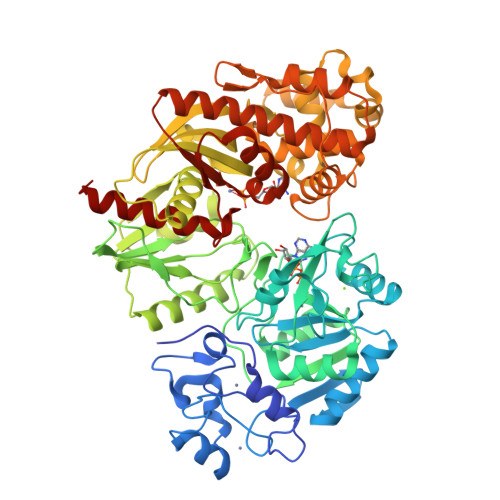
[NiFe]-hydrogenases are multimeric proteins. The large subunit contains the NiFe(CN)(2)CO bimetallic active center and the small subunit contains Fe-S clusters. Biosynthesis and assembly of the NiFe(CN)(2)CO active center requires six Hyp accessory proteins. The synthesis of the CN(-) ligands is catalyzed by the combined actions of HypF and HypE using carbamoylphosphate as a substrate. We report the structure of Escherichia coli HypF(92-750) lacking the N-terminal acylphosphatase domain. HypF(92-750) comprises the novel Zn-finger domain, the nucleotide-binding YrdC-like domain, and the Kae1-like universal domain, also binding a nucleotide and a Zn(2+) ion. The two nucleotide-binding sites are sequestered in an internal cavity, facing each other and separated by ∼14 Å. The YrdC-like domain converts carbamoyl moiety to a carbamoyl adenylate intermediate, which is channeled to the Kae1-like domain. Mutations within either nucleotide-binding site compromise hydrogenase maturation but do not affect the carbamoylphosphate phosphatase activity.
Organizational Affiliation:
Department of Biochemistry, McGill University, Montreal, QC H3G 1Y6, Canada.