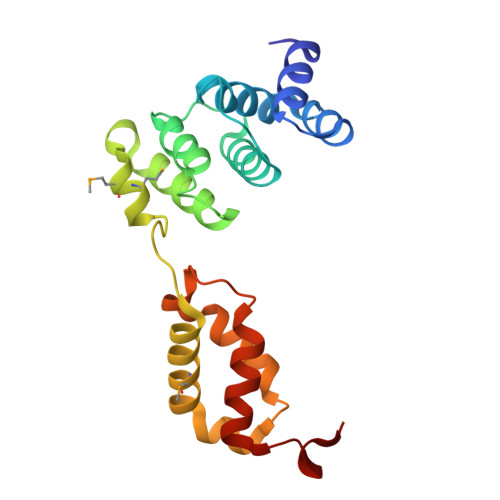
Structural conservation of an ancient tRNA sensor in eukaryotic glutaminyl-tRNA synthetase.
Grant, T.D., Snell, E.H., Luft, J.R., Quartley, E., Corretore, S., Wolfley, J.R., Snell, M.E., Hadd, A., Perona, J.J., Phizicky, E.M., Grayhack, E.J.(2012) Nucleic Acids Res 40: 3723-3731
- PubMed: 22180531
- DOI: https://doi.org/10.1093/nar/gkr1223
- Primary Citation of Related Structures:
3TL4 - PubMed Abstract:
In all organisms, aminoacyl tRNA synthetases covalently attach amino acids to their cognate tRNAs. Many eukaryotic tRNA synthetases have acquired appended domains, whose origin, structure and function are poorly understood. The N-terminal appended domain (NTD) of glutaminyl-tRNA synthetase (GlnRS) is intriguing since GlnRS is primarily a eukaryotic enzyme, whereas in other kingdoms Gln-tRNA(Gln) is primarily synthesized by first forming Glu-tRNA(Gln), followed by conversion to Gln-tRNA(Gln) by a tRNA-dependent amidotransferase. We report a functional and structural analysis of the NTD of Saccharomyces cerevisiae GlnRS, Gln4. Yeast mutants lacking the NTD exhibit growth defects, and Gln4 lacking the NTD has reduced complementarity for tRNA(Gln) and glutamine. The 187-amino acid Gln4 NTD, crystallized and solved at 2.3 Å resolution, consists of two subdomains, each exhibiting an extraordinary structural resemblance to adjacent tRNA specificity-determining domains in the GatB subunit of the GatCAB amidotransferase, which forms Gln-tRNA(Gln). These subdomains are connected by an apparent hinge comprised of conserved residues. Mutation of these amino acids produces Gln4 variants with reduced affinity for tRNA(Gln), consistent with a hinge-closing mechanism proposed for GatB recognition of tRNA. Our results suggest a possible origin and function of the NTD that would link the phylogenetically diverse mechanisms of Gln-tRNA(Gln) synthesis.
Organizational Affiliation:
Hauptman-Woodward Medical Research Institute, Buffalo, NY 14203, USA.