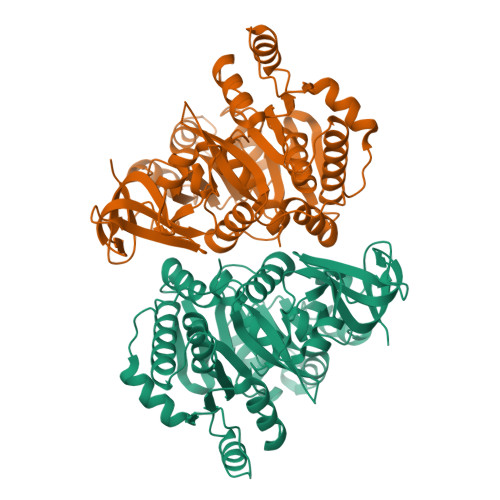
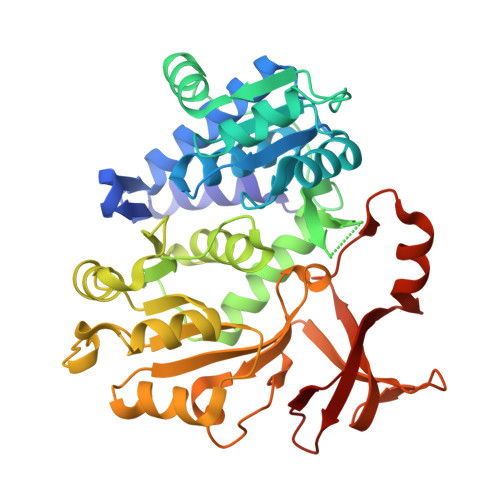
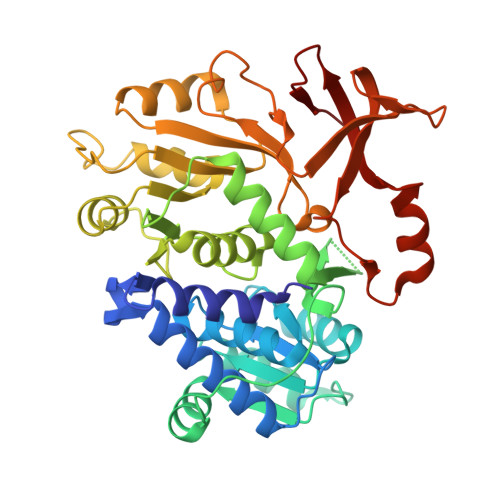
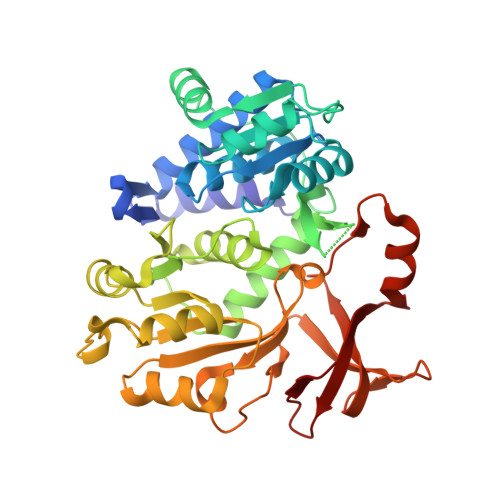
Molecular basis of the functional divergence of fatty acyl-AMP ligase biosynthetic enzymes of Mycobacterium tuberculosis.
Goyal, A., Verma, P., Anandhakrishnan, M., Gokhale, R.S., Sankaranarayanan, R.(2012) J Mol Biology 416: 221-238
- PubMed: 22206988
- DOI: https://doi.org/10.1016/j.jmb.2011.12.031
- Primary Citation of Related Structures:
3T5A, 3T5B, 3T5C - PubMed Abstract:
Activation of fatty acids as acyl-adenylates by fatty acyl-AMP ligases (FAALs) in Mycobacterium tuberculosis is a variant of a classical theme that involves formation of acyl-CoA (coenzyme A) by fatty acyl-CoA ligases (FACLs). Here, we show that FAALs and FACLs possess similar structural fold and substrate specificity determinants, and the key difference is the absence of a unique insertion sequence in FACL13 structure. A systematic analysis shows a conserved hydrophobic anchorage of the insertion motif across several FAALs. Strikingly, mutagenesis of two phenylalanine residues, which are part of the anchorage, to alanine converts FAAL32 to FACL32. This insertion-based in silico analysis suggests the presence of FAAL homologues in several other non-mycobacterial genomes including eukaryotes. The work presented here establishes an elegant mechanism wherein an insertion sequence drives the functional divergence of FAALs from canonical FACLs.
Organizational Affiliation:
Centre for Cellular and Molecular Biology, Council of Scientific and Industrial Research, Hyderabad 500 007, India.