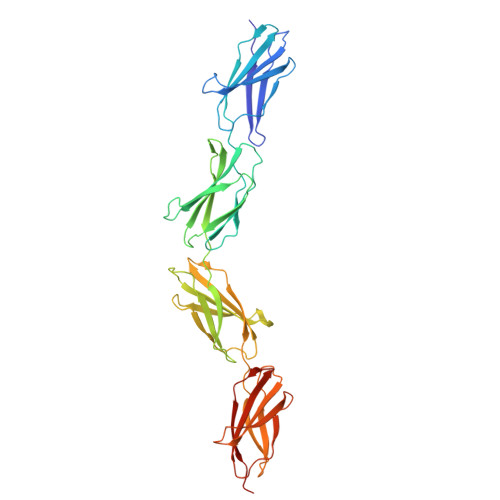
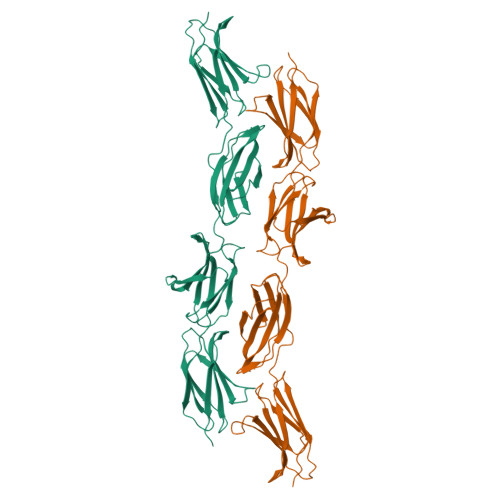
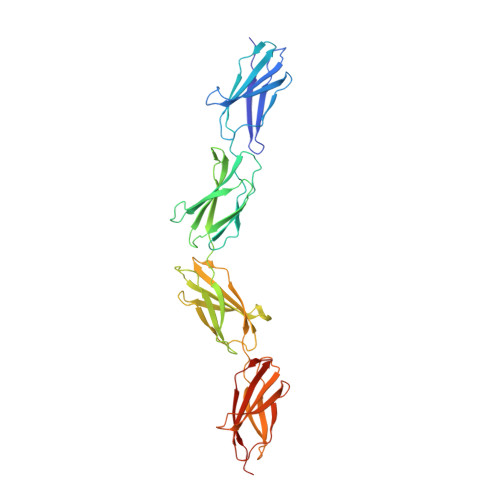
Extra-domain B in oncofetal fibronectin structurally promotes fibrillar head-to-tail dimerization of extracellular matrix protein.
Schiefner, A., Gebauer, M., Skerra, A.(2012) J Biological Chem 287: 17578-17588
- PubMed: 22442152
- DOI: https://doi.org/10.1074/jbc.M111.303131
- Primary Citation of Related Structures:
3T1W - PubMed Abstract:
The type III extra-domain B (ED-B) is specifically spliced into fibronectin (Fn) during embryogenesis and neoangiogenesis, including many cancers. The x-ray structure of the recombinant four-domain fragment Fn(III)7B89 reveals a tightly associated, extended head-to-tail dimer, which is stabilized via pair-wise shape and charge complementarity. A tendency toward ED-B-dependent dimer formation in solution was supported by size exclusion chromatography and analytical ultracentrifugation. When amending the model with the known three-dimensional structure of the Fn(III)10 domain, its RGD loop as well as the adhesion synergy region in Fn(III)9-10 become displayed on the same face of the dimer; this should allow simultaneous binding of at least two integrins and, thus, receptor clustering on the cell surface and intracellular signaling. Insertion of ED-B appears to stabilize overall head-to-tail dimerization of two separate Fn chains, which, together with alternating homodimer formation via disulfide bridges at the C-terminal Fn tail, should lead to the known macromolecular fibril formation.
Organizational Affiliation:
Munich Center for Integrated Protein Science (CIPS-M) and Lehrstuhl für Biologische Chemie, Technische Universität München, 85350 Freising-Weihenstephan, Germany.