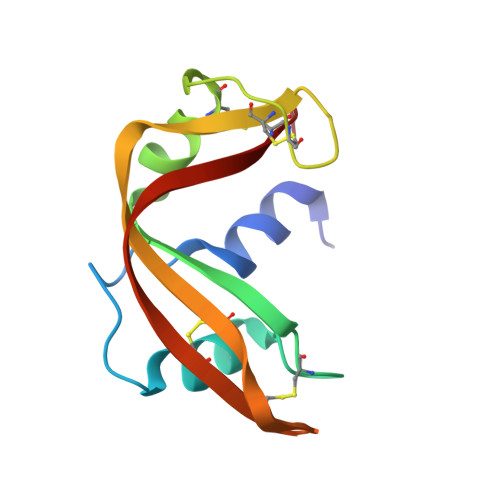
Structural changes that accompany the reduced catalytic efficiency of two semisynthetic ribonuclease analogs.
deMel, V.S., Martin, P.D., Doscher, M.S., Edwards, B.F.(1992) J Biol Chem 267: 247-256
- PubMed: 1730593
- Primary Citation of Related Structures:
3SRN, 4SRN - PubMed Abstract:
The structures of two catalytically defective semi-synthetic RNases obtained by replacing aspartic acid 121 with asparagine or alanine have been determined and refined at a resolution of 2.0 A (R = 0.186 and 0.172, respectively). When these structures are compared with the refined 1.8-A structure (R = 0.204) of the fully active aspartic acid-containing enzyme (Martin, P.D., Doscher, M.S., and Edwards, B. F. P. (1987) J. Biol. Chem. 262, 15930-15938), numerous and widespread changes, much greater in number and magnitude than the small structural variations noted previously between the semisynthetic complex and RNase A, are found to have occurred. These changes include the movement of the loop containing residues 65-72 away from the active site, a more or less generalized relocation of crystallographically bound water molecules, and a number of rearrangements in the hydrogen bonding network at the active site. Most changes are far removed from the immediate site of the modifications and are distributed essentially throughout the molecule. The details of many of these changes are unique to each analog. In the asparagine analog, a destabilization in the positioning of active site residue His-119 also appears to have occurred.
Organizational Affiliation:
Department of Biochemistry, Wayne State University School of Medicine, Detroit, Michigan 48201.