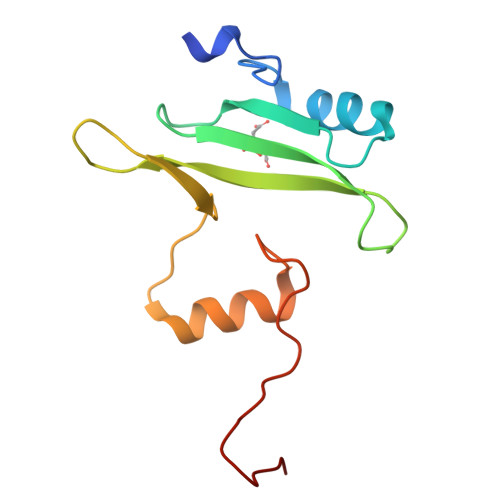
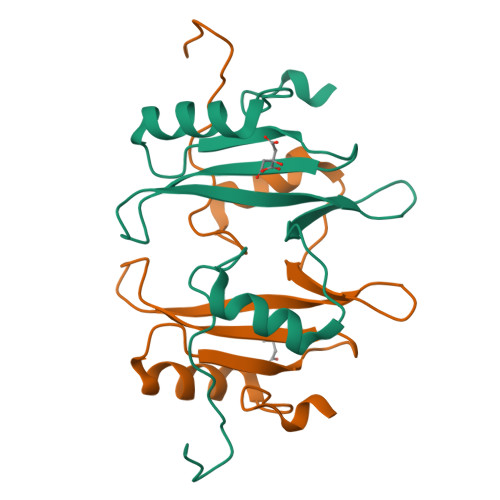
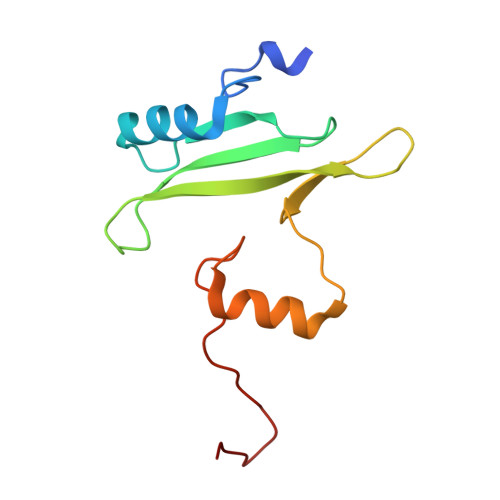
Structure of the interleukin-2 tyrosine kinase Src homology 2 domain; comparison between X-ray and NMR-derived structures.
Joseph, R.E., Ginder, N.D., Hoy, J.A., Nix, J.C., Fulton, D.B., Honzatko, R.B., Andreotti, A.H.(2012) Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 145-153
- PubMed: 22297986
- DOI: https://doi.org/10.1107/S1744309111049761
- Primary Citation of Related Structures:
3S9K - PubMed Abstract:
The crystal structure of the interleukin-2 tyrosine kinase Src homology domain (Itk SH2) is described and it is found that unlike in studies of this domain using NMR spectroscopy, cis-trans-prolyl isomerization is not readily detected in the crystal structure. Based on similarities between the Itk SH2 crystal form and the cis form of the Itk SH2 NMR structure, it is concluded that it is likely that the prolyl imide bond at least in part adopts the cis conformation in the crystal form. However, the lack of high-resolution data and the dynamic nature of the proline-containing loop mean that the precise imide-bond conformation cannot be determined and prolyl cis-trans isomerization in the crystal cannot be ruled out. Given the preponderance of structures that have been solved by X-ray crystallography in the Protein Data Bank, this result supports the notion that prolyl isomerization in folded proteins has been underestimated among known structures. Interestingly, while the precise status of the proline residue is ambiguous, Itk SH2 crystallizes as a domain-swapped dimer. The domain-swapped structure of Itk SH2 is similar to the domain-swapped SH2 domains of Grb2 and Nck, with domain swapping occurring at the β-meander region of all three SH2 domains. Thus, for Itk SH2 structural analysis by NMR spectroscopy and X-ray crystallography revealed very different structural features: proline isomerization versus domain-swapped dimerization, respectively.
Organizational Affiliation:
Department of Biochemistry, Biophysics and Molecular Biology, Iowa State University, Ames, IA 50011, USA.