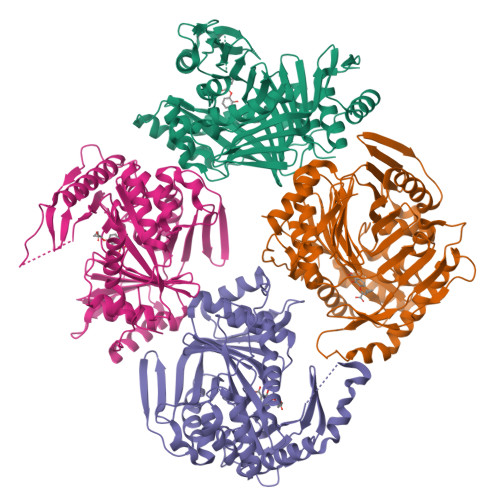
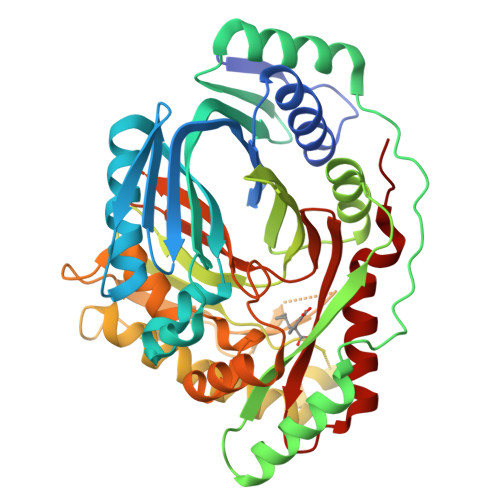
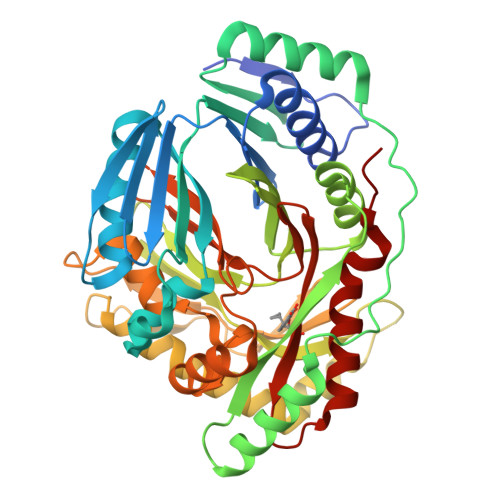
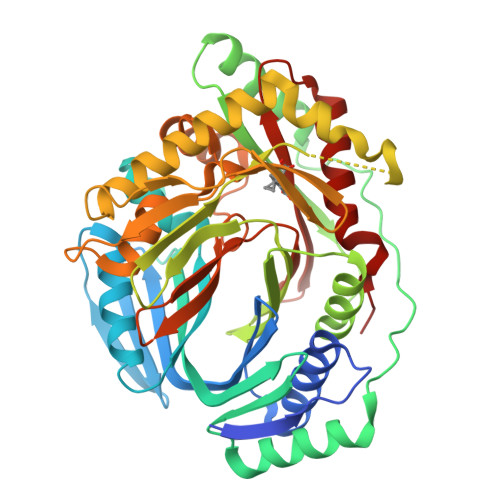
Implications of binding mode and active site flexibility for inhibitor potency against the salicylate synthase from Mycobacterium tuberculosis
Chi, G., Manos-Turvey, A., O'Connor, P.D., Johnston, J.M., Evans, G.L., Baker, E.N., Payne, R.J., Lott, J.S., Bulloch, E.M.(2012) Biochemistry 51: 4868-4879
- PubMed: 22607697
- DOI: https://doi.org/10.1021/bi3002067
- Primary Citation of Related Structures:
3RV6, 3RV7, 3RV8, 3RV9, 3ST6, 3VEH - PubMed Abstract:
MbtI is the salicylate synthase that catalyzes the first committed step in the synthesis of the iron chelating compound mycobactin in Mycobacterium tuberculosis. We previously developed a series of aromatic inhibitors against MbtI based on the reaction intermediate for this enzyme, isochorismate. The most potent of these inhibitors had hydrophobic substituents, ranging in size from a methyl to a phenyl group, appended to the terminal alkene of the enolpyruvyl group. These compounds exhibited low micromolar inhibition constants against MbtI and were at least an order of magnitude more potent than the parental compound for the series, which carries a native enolpyruvyl group. In this study, we sought to understand how the substituted enolpyruvyl group confers greater potency, by determining cocrystal structures of MbtI with six inhibitors from the series. A switch in binding mode at the MbtI active site is observed for inhibitors carrying a substituted enolpyruvyl group, relative to the parental compound. Computational studies suggest that the change in binding mode, and higher potency, is due to the effect of the substituents on the conformational landscape of the core inhibitor structure. The crystal structures and fluorescence-based thermal shift assays indicate that substituents larger than a methyl group are accommodated in the MbtI active site through significant but localized flexibility in the peptide backbone. These findings have implications for the design of improved inhibitors of MbtI, as well as other chorismate-utilizing enzymes from this family.
Organizational Affiliation:
School of Biological Sciences and Maurice Wilkins Centre for Molecular Biodiscovery, The University of Auckland, 3 Symonds Street, Private Bag 92019, Auckland 1142, New Zealand.