Acoustically Mounted Microcrystals Yield High-Resolution X-ray Structures.
Soares, A.S., Engel, M.A., Stearns, R., Datwani, S., Olechno, J., Ellson, R., Skinner, J.M., Allaire, M., Orville, A.M.(2011) Biochemistry 50: 4399-4401
- PubMed: 21542590
- DOI: https://doi.org/10.1021/bi200549x
- Primary Citation of Related Structures:
3RTO - PubMed Abstract:
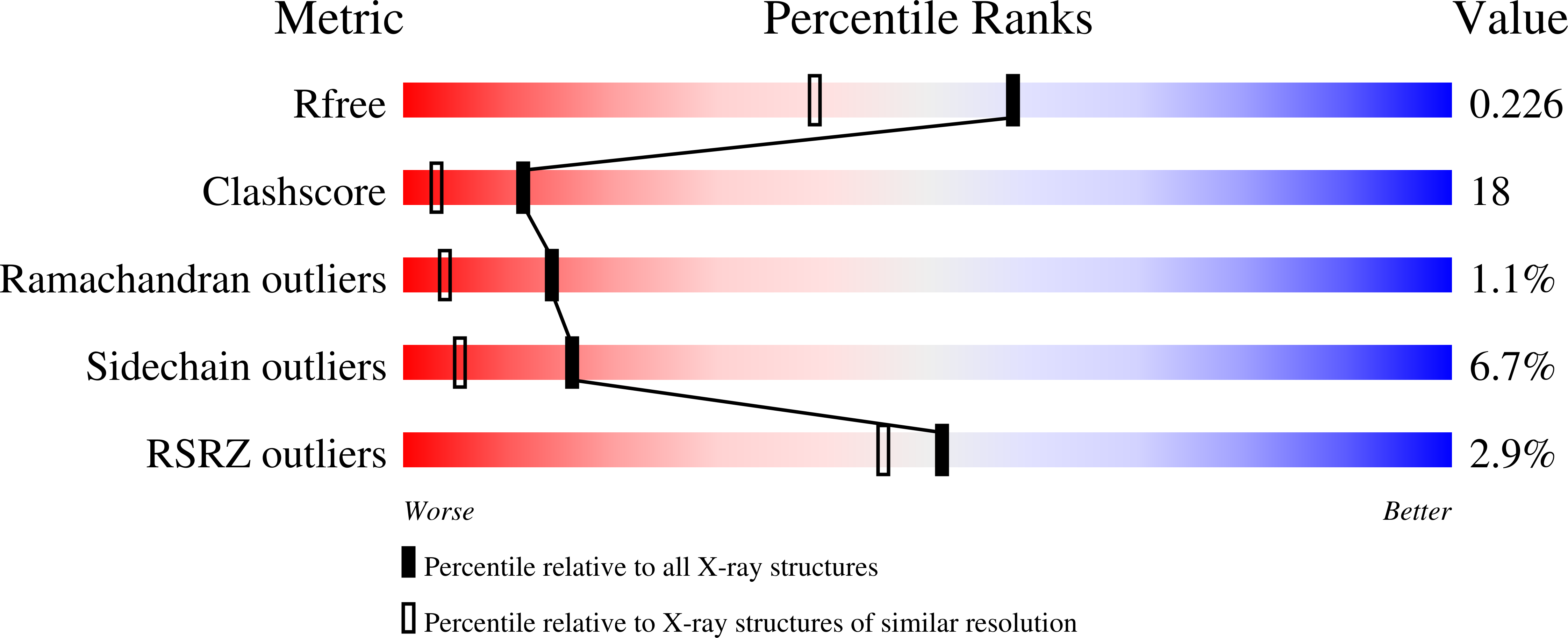
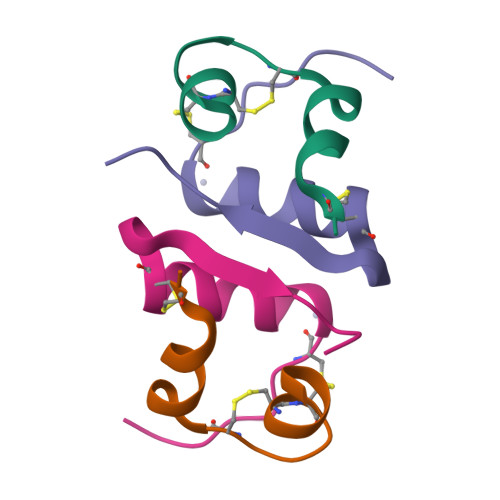
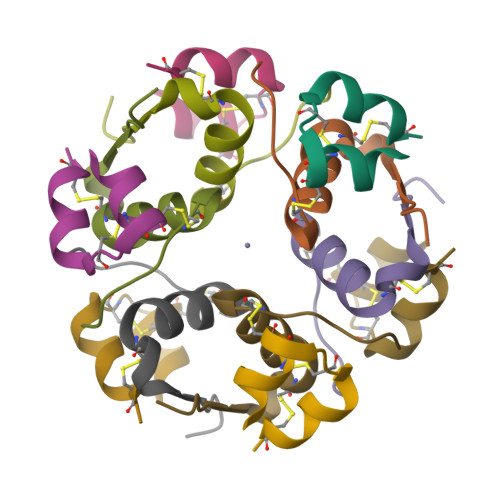
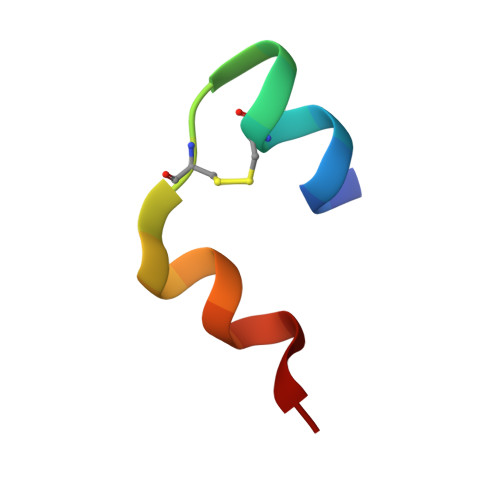
We demonstrate a general strategy for determining structures from showers of microcrystals. It uses acoustic droplet ejection to transfer 2.5 nL droplets from the surface of microcrystal slurries, through the air, onto mounting micromesh pins. Individual microcrystals are located by raster-scanning a several-micrometer X-ray beam across the cryocooled micromeshes. X-ray diffraction data sets merged from several micrometer-sized crystals are used to determine 1.8 Ǻ resolution crystal structures.
Organizational Affiliation:
Biology Department, Brookhaven National Laboratory, Upton, NY 11973-5000, USA. soares@bnl.gov