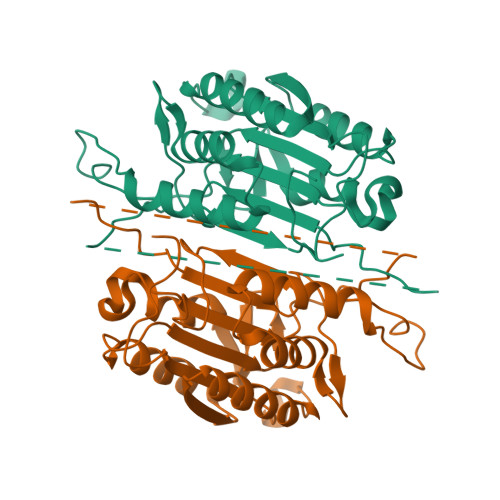
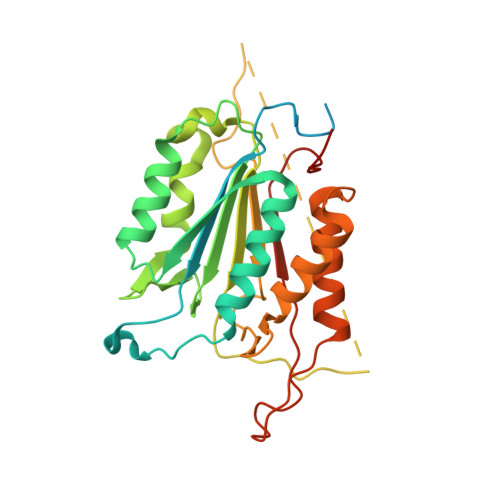
A designed redox-controlled caspase.
Witkowski, W.A., Hardy, J.A.(2011) Protein Sci 20: 1421-1431
- PubMed: 21674661
- DOI: https://doi.org/10.1002/pro.673
- Primary Citation of Related Structures:
3R5K - PubMed Abstract:
Caspases are a powerful class of cysteine proteases. Introduction of activated caspases in healthy or cancerous cells results in induction of apoptotic cell death. In this study, we have designed and characterized a version of caspase-7 that can be inactivated under oxidizing extracellular conditions and then reactivated under reducing intracellular conditions. This version of caspase-7 is allosterically inactivated when two of the substrate-binding loops are locked together via an engineered disulfide. When this disulfide is reduced, the protein regains its full function. The inactive loop-locked version of caspase-7 can be readily observed by immunoblotting and mass spectrometry. The reduced and reactivated form of the enzyme observed crystallographically is the first caspase-7 structure in which the substrate-binding groove is properly ordered even in the absence of an active-site ligand. In the reactivated structure, the catalytic-dyad cysteine-histidine are positioned 3.5 Å apart in an orientation that is capable of supporting catalysis. This redox-controlled version of caspase-7 is particularly well suited for targeted cell death in concert with redox-triggered delivery vehicles.
Organizational Affiliation:
Department of Chemistry, University of Massachusetts Amherst, Amherst, Massachusetts 01003, USA.