The X-ray crystallographic structure and specificity profile of HAD superfamily phosphohydrolase BT1666: Comparison of paralogous functions in B. thetaiotaomicron.
Lu, Z., Dunaway-Mariano, D., Allen, K.N.(2011) Proteins 79: 3099-3107
- PubMed: 21989931
- DOI: https://doi.org/10.1002/prot.23137
- Primary Citation of Related Structures:
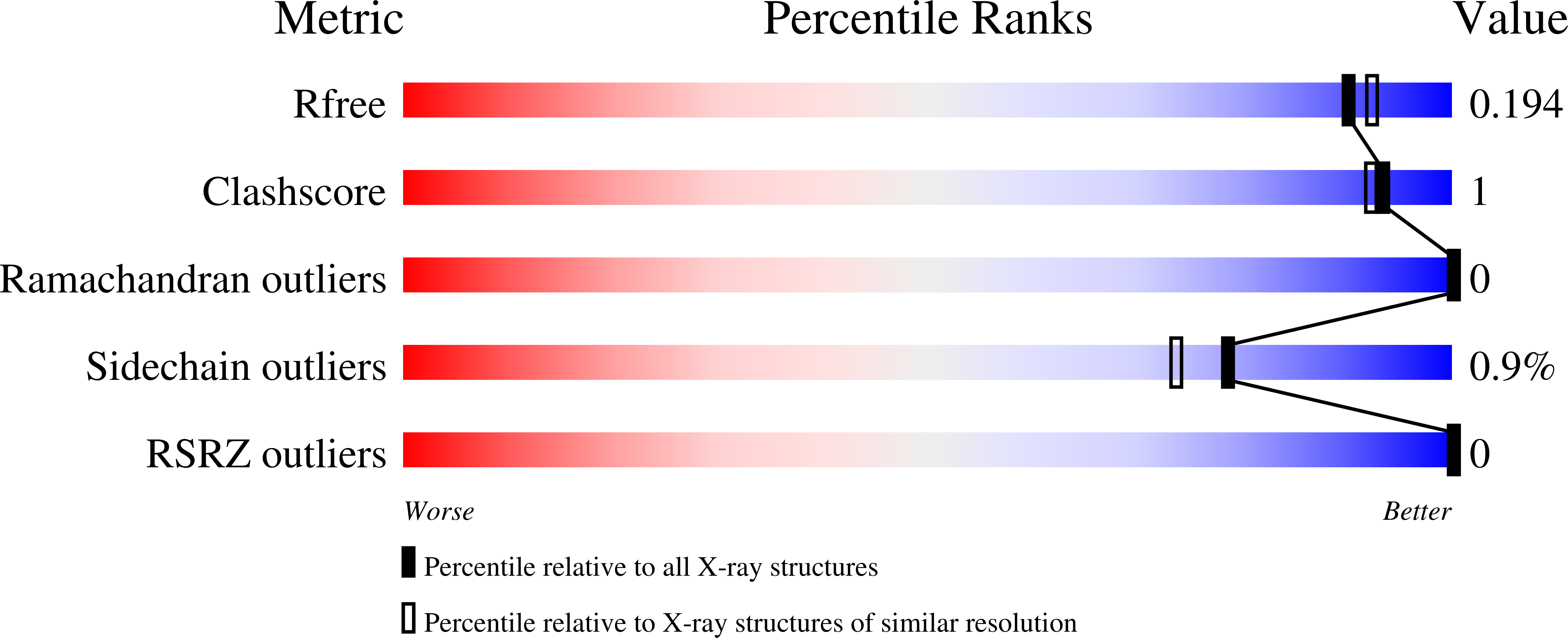
3R4C - PubMed Abstract:
Analysis of the haloalkanoate dehalogenase superfamily (HADSF) has uncovered homologues occurring within the same organism that are found to possess broad, overlapping substrate specificities, and low catalytic efficiencies. Here we compare the HADSF phosphatase BT1666 from Bacteroides thetaiotaomicron VPI-5482 to a homologue with high sequence identity (40%) from the same organism BT4131, a known hexose-phosphate phosphatase. The goal is to find whether these enzymes represent duplicated versus paralogous activities. The X-ray crystal structure of BT1666 was determined to 1.82 Å resolution. Superposition of the BT1666 and BT4131 structures revealed a conserved fold and identical active sites suggestive of a common physiological substrate. The steady-state kinetic constants for BT1666 were determined for a diverse panel of phosphorylated metabolites to define its substrate specificity profile and overall level of catalytic efficiency. Whereas BT1666 and BT4131 are both promiscuous, their substrate specificity profiles are distinct. The catalytic efficiency of BT1666 (k(cat) /K(m) = 4.4 × 10(2) M(-1) s(-1) for the best substrate fructose 1,6-(bis)phosphate) is an order of magnitude less than that of BT4131 (k(cat) /K(m) = 6.7 × 10(3) M(-1) s(-1) for 2-deoxyglucose 6-phosphate). The seemingly identical active-site structures point to sequence variation outside the active site causing differences in conformational dynamics or subtle catalytic positioning effects that drive the divergence in catalytic efficiency and selectivity. The overlapping substrate profiles may be understood in terms of differential regulation of expression of the two enzymes or a conferred advantage in metabolic housekeeping functions by having a larger range of possible metabolites as substrates.
Organizational Affiliation:
Department of Chemistry, Boston University, Boston, Massachusetts 02215, USA.