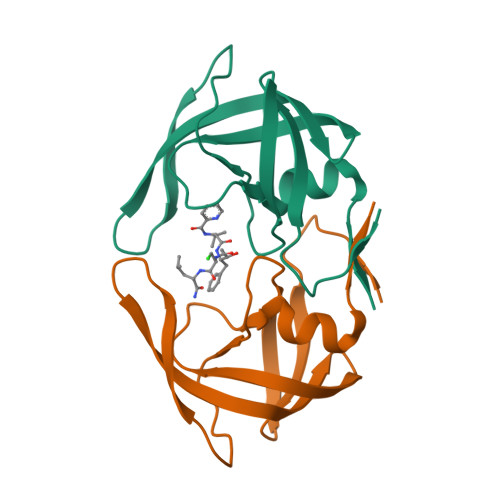
Crystal structures of multidrug-resistant HIV-1 protease in complex with two potent anti-malarial compounds.
Yedidi, R.S., Liu, Z., Wang, Y., Brunzelle, J.S., Kovari, I.A., Woster, P.M., Kovari, L.C., Gupta, D.(2012) Biochem Biophys Res Commun 421: 413-417
- PubMed: 22469467
- DOI: https://doi.org/10.1016/j.bbrc.2012.03.096
- Primary Citation of Related Structures:
3R0W, 3R0Y - PubMed Abstract:
Two potent inhibitors (compounds 1 and 2) of malarial aspartyl protease, plasmepsin-II, were evaluated against wild type (NL4-3) and multidrug-resistant clinical isolate 769 (MDR) variants of human immunodeficiency virus type-1 (HIV-1) aspartyl protease. Enzyme inhibition assays showed that both 1 and 2 have better potency against NL4-3 than against MDR protease. Crystal structures of MDR protease in complex with 1 and 2 were solved and analyzed. Crystallographic analysis revealed that the MDR protease exhibits a typical wide-open conformation of the flaps (Gly48 to Gly52) causing an overall expansion in the active site cavity, which, in turn caused unstable binding of the inhibitors. Due to the expansion of the active site cavity, both compounds showed loss of direct contacts with the MDR protease compared to the docking models of NL4-3. Multiple water molecules showed a rich network of hydrogen bonds contributing to the stability of the ligand binding in the distorted binding pockets of the MDR protease in both crystal structures. Docking analysis of 1 and 2 showed a decrease in the binding affinity for both compounds against MDR supporting our structure-function studies. Thus, compounds 1 and 2 show promising inhibitory activity against HIV-1 protease variants and hence are good candidates for further development to enhance their potency against NL4-3 as well as MDR HIV-1 protease variants.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, School of Medicine, Wayne State University, 540 E. Canfield Avenue, Detroit, MI 48201, USA. yedidirs@mail.nih.gov