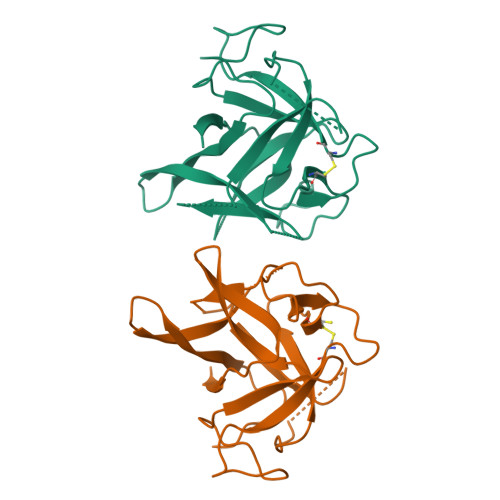
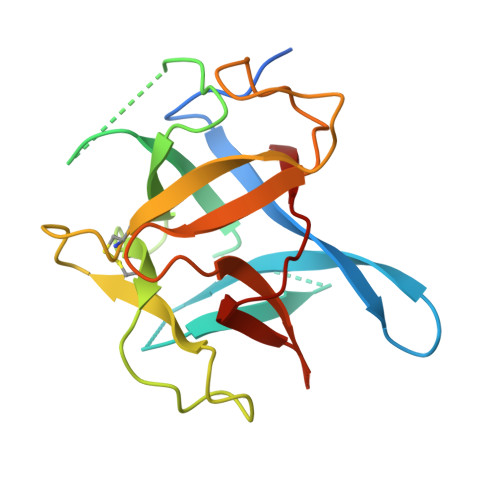
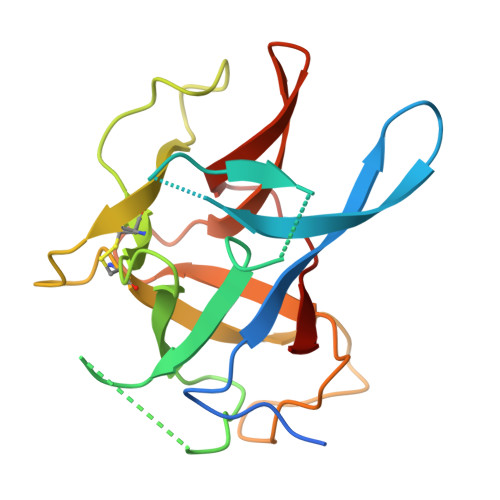
The deletion of exon 3 in the cardiac ryanodine receptor is rescued by beta strand switching.
Lobo, P.A., Kimlicka, L., Tung, C.C., Van Petegem, F.(2011) Structure 19: 790-798
- PubMed: 21645850
- DOI: https://doi.org/10.1016/j.str.2011.03.016
- Primary Citation of Related Structures:
3QR5 - PubMed Abstract:
Mutations in the cardiac Ryanodine Receptor (RYR2) are linked to triggered arrhythmias. Removal of exon 3 results in a severe form of catecholaminergic polymorphic ventricular tachycardia (CPVT). This exon encodes secondary structure elements that are crucial for folding of the N-terminal domain (NTD), raising the question of why the deletion is neither lethal nor confers a loss of function. We determined the 2.3 Å crystal structure of the NTD lacking exon 3. The removal causes a structural rescue whereby a flexible loop inserts itself into the β trefoil domain and increases thermal stability. The exon 3 deletion is not tolerated in the corresponding RYR1 domain. The rescue shows a novel mechanism by which RYR2 channels can adjust their Ca²⁺ release properties through altering the structure of the NTD. Despite the rescue, the deletion affects interfaces with other RYR2 domains. We propose that relative movement of the NTD is allosterically coupled to the pore region.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of British Columbia, 2350 Health Sciences Mall, room 2.320, Vancouver, BC V6T1Z3, Canada.