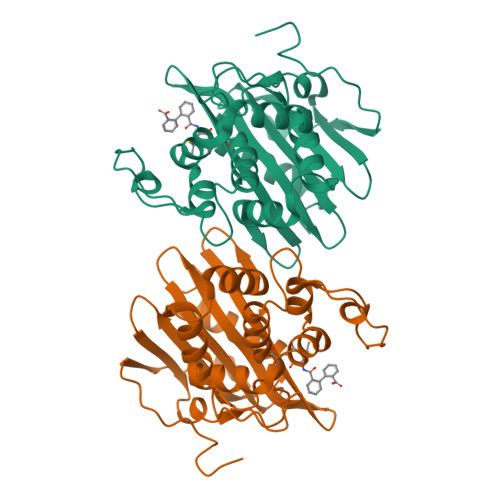
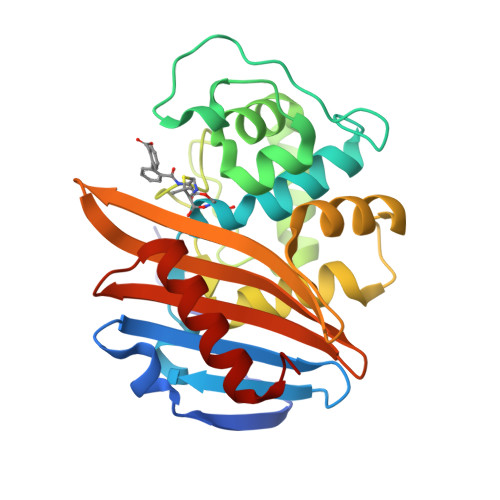
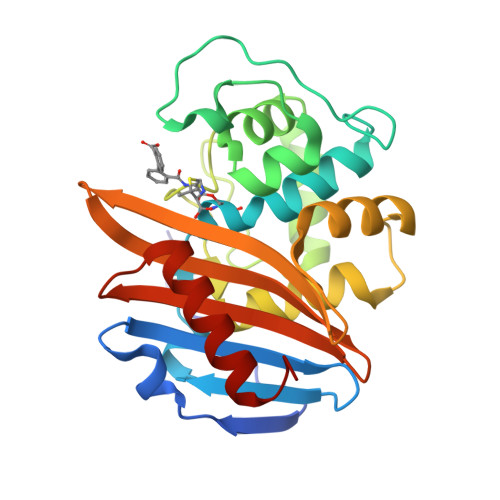
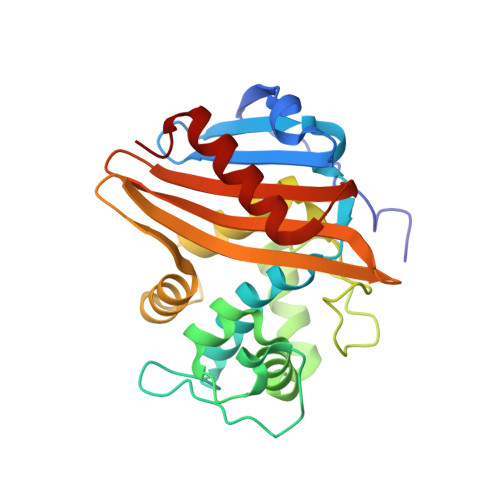
Lysine Nzeta-decarboxylation switch and activation of the beta-lactam sensor domain of BlaR1 protein of methicillin-resistant Staphylococcus aureus.
Borbulevych, O., Kumarasiri, M., Wilson, B., Llarrull, L.I., Lee, M., Hesek, D., Shi, Q., Peng, J., Baker, B.M., Mobashery, S.(2011) J Biological Chem 286: 31466-31472
- PubMed: 21775440
- DOI: https://doi.org/10.1074/jbc.M111.252189
- Primary Citation of Related Structures:
3Q7V, 3Q7Z, 3Q81, 3Q82 - PubMed Abstract:
The integral membrane protein BlaR1 of methicillin-resistant Staphylococcus aureus senses the presence of β-lactam antibiotics in the milieu and transduces the information to the cytoplasm, where the biochemical events that unleash induction of antibiotic resistance mechanisms take place. We report herein by two-dimensional and three-dimensional NMR experiments of the sensor domain of BlaR1 in solution and by determination of an x-ray structure for the apo protein that Lys-392 of the antibiotic-binding site is posttranslationally modified by N(ζ)-carboxylation. Additional crystallographic and NMR data reveal that on acylation of Ser-389 by antibiotics, Lys-392 experiences N(ζ)-decarboxylation. This unique process, termed the lysine N(ζ)-decarboxylation switch, arrests the sensor domain in the activated ("on") state, necessary for signal transduction and all the subsequent biochemical processes. We present structural information on how this receptor activation process takes place, imparting longevity to the antibiotic-receptor complex that is needed for the induction of the antibiotic-resistant phenotype in methicillin-resistant S. aureus.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, Indiana 46556, USA.