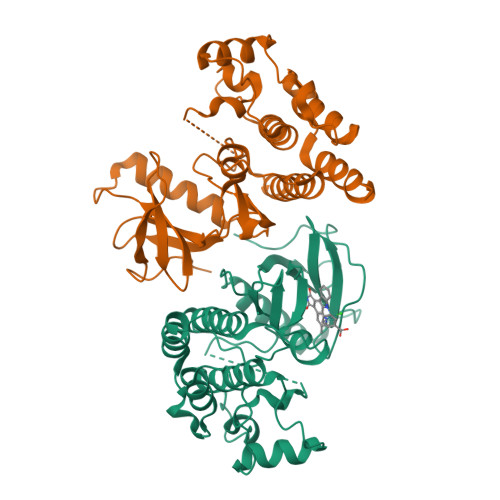
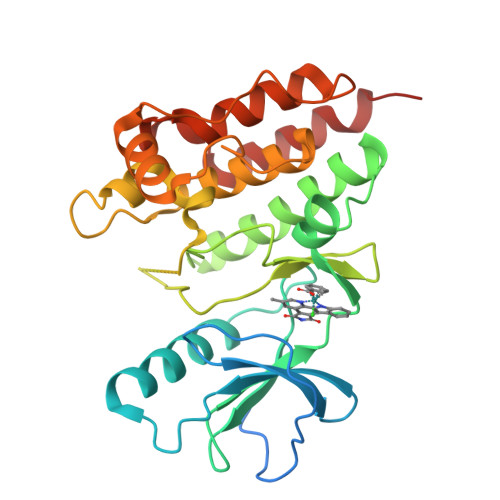
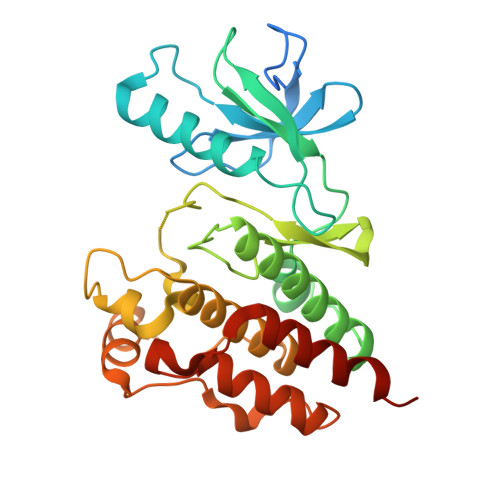
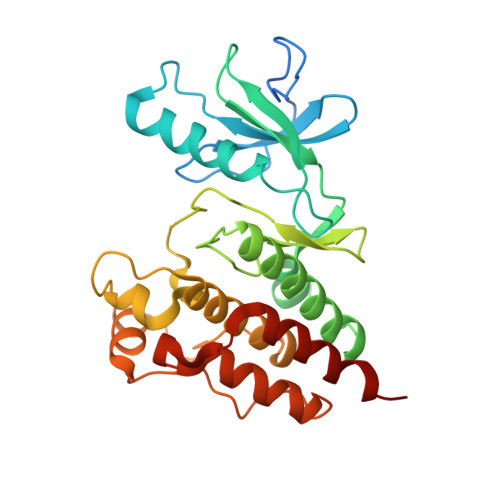
The crystal structure of BRAF in complex with an organoruthenium inhibitor reveals a mechanism for inhibition of an active form of BRAF kinase.
Xie, P., Streu, C., Qin, J., Bregman, H., Pagano, N., Meggers, E., Marmorstein, R.(2009) Biochemistry 48: 5187-5198
- PubMed: 19371126
- DOI: https://doi.org/10.1021/bi802067u
- Primary Citation of Related Structures:
3Q4C - PubMed Abstract:
Substitution mutations in the BRAF serine/threonine kinase are found in a variety of human cancers. Such mutations occur in approximately 70% of human malignant melanomas, and a single hyperactivating V600E mutation is found in the activation segment of the kinase domain and accounts for more than 90% of these mutations. Given this correlation, the molecular mechanism for BRAF regulation as well as oncogenic activation has attracted considerable interest, and activated forms of BRAF, such as BRAF(V600E), have become attractive targets for small molecule inhibition. Here we report on the identification and subsequent optimization of a potent BRAF inhibitor, CS292, based on an organometallic kinase inhibitor scaffold. A cocrystal structure of CS292 in complex with the BRAF kinase domain reveals that CS292 binds to the ATP binding pocket of the kinase and is an ATP competitive inhibitor. The structure of the kinase-inhibitor complex also demonstrates that CS292 binds to BRAF in an active conformation and suggests a mechanism for regulation of BRAF by phosphorylation and BRAF(V600E) oncogene-induced activation. The structure of CS292 bound to the active form of the BRAF kinase also provides a novel scaffold for the design of BRAF(V600E) oncogene selective BRAF inhibitors for therapeutic application.
Organizational Affiliation:
The Wistar Institute, Philadelphia, Pennsylvania 19104-6323, USA.