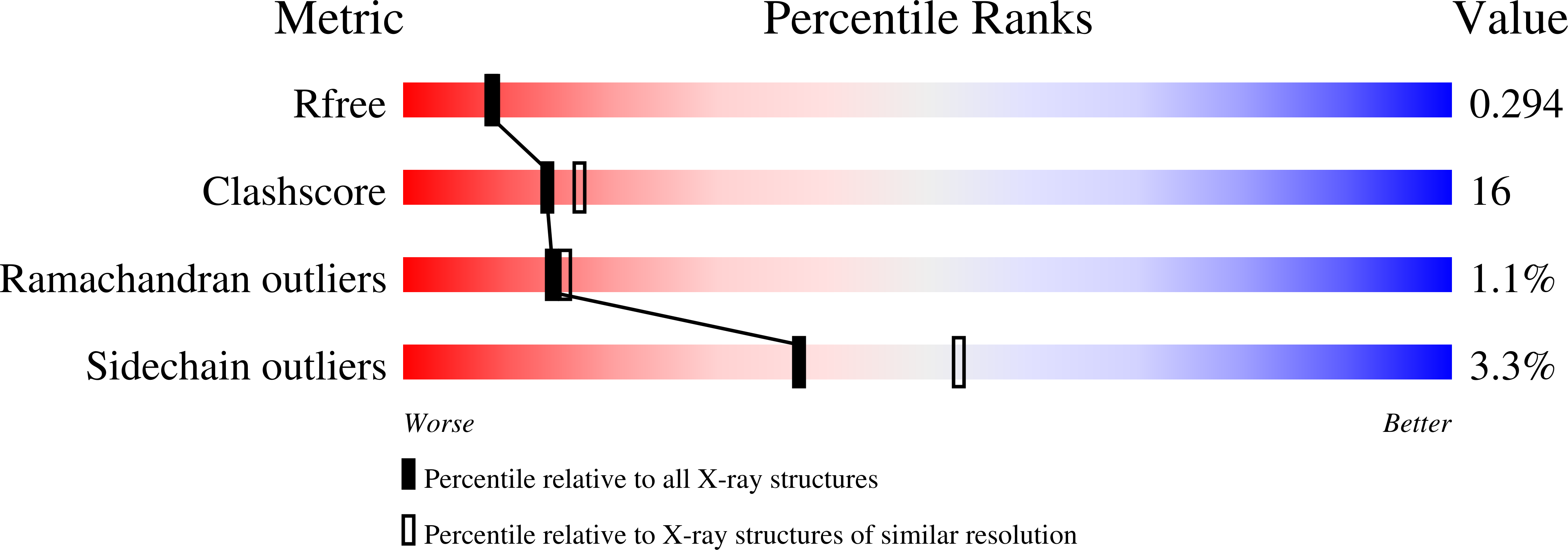
Insulin fibrillation is the Janus face of induced fit. A chiral clamp stabilizes the native state at the expense of activity
Hua, Q.X., Wan, Z.L., Huang, K., Hu, S.Q., Phillip, N.F., Jia, W.H., Whittingham, J., Dodson, G.G., Katsoyannis, P.G., Weiss, M.A.To be published.