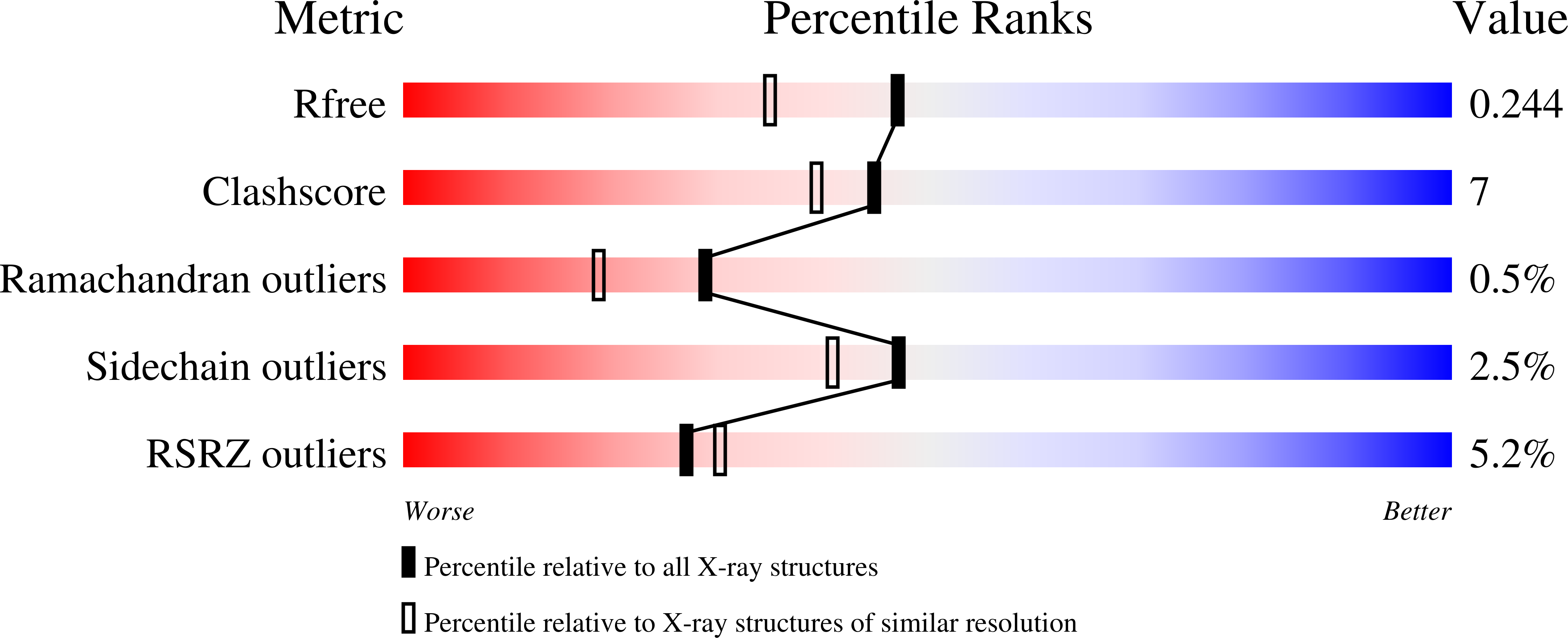
Cyclodipeptide synthases, a family of class-I aminoacyl-tRNA synthetase-like enzymes involved in non-ribosomal peptide synthesis.
Sauguet, L., Moutiez, M., Li, Y., Belin, P., Seguin, J., Le Du, M.H., Thai, R., Masson, C., Fonvielle, M., Pernodet, J.L., Charbonnier, J.B., Gondry, M.(2011) Nucleic Acids Res 39: 4475-4489
- PubMed: 21296757
- DOI: https://doi.org/10.1093/nar/gkr027
- Primary Citation of Related Structures:
3OQV - PubMed Abstract:
Cyclodipeptide synthases (CDPSs) belong to a newly defined family of enzymes that use aminoacyl-tRNAs (aa-tRNAs) as substrates to synthesize the two peptide bonds of various cyclodipeptides, which are the precursors of many natural products with noteworthy biological activities. Here, we describe the crystal structure of AlbC, a CDPS from Streptomyces noursei. The AlbC structure consists of a monomer containing a Rossmann-fold domain. Strikingly, it is highly similar to the catalytic domain of class-I aminoacyl-tRNA synthetases (aaRSs), especially class-Ic TyrRSs and TrpRSs. AlbC contains a deep pocket, highly conserved among CDPSs. Site-directed mutagenesis studies indicate that this pocket accommodates the aminoacyl moiety of the aa-tRNA substrate in a way similar to that used by TyrRSs to recognize their tyrosine substrates. These studies also suggest that the tRNA moiety of the aa-tRNA interacts with AlbC via at least one patch of basic residues, which is conserved among CDPSs but not present in class-Ic aaRSs. AlbC catalyses its two-substrate reaction via a ping-pong mechanism with a covalent intermediate in which L-Phe is shown to be transferred from Phe-tRNA(Phe) to an active serine. These findings provide insight into the molecular bases of the interactions between CDPSs and their aa-tRNAs substrates, and the catalytic mechanism used by CDPSs to achieve the non-ribosomal synthesis of cyclodipeptides.
Organizational Affiliation:
CEA, IBITECS, Service d'Ingénierie Moléculaire des Protéines, F-91191 Gif-sur-Yvette, France.