Crystal structure of peptidyl-tRNA hydrolase from Escherichia Coli, I222 crystal form
Lam, R., McGrath, T.E., Romanov, V., Gothe, S.A., Peddi, S.R., Razumova, E., Lipman, R.S., Branstrom, A.A., Chirgadze, N.Y.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peptidyl-tRNA hydrolase | 211 | Escherichia coli B354 | Mutation(s): 0 Gene Names: ECEG_00530, ECs1709, pth EC: 3.1.1.29 | 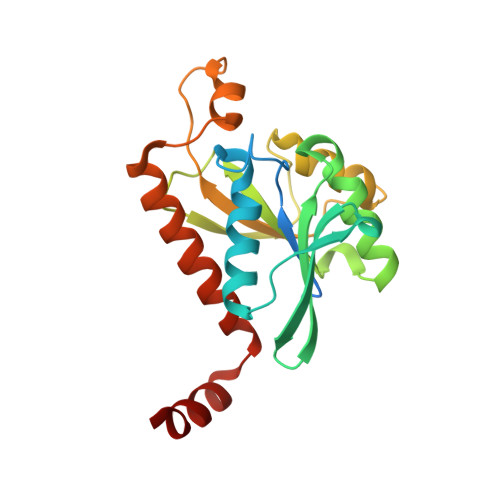 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 74.38 | α = 90 |
| b = 96.96 | β = 90 |
| c = 207.678 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| StructureStudio | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |