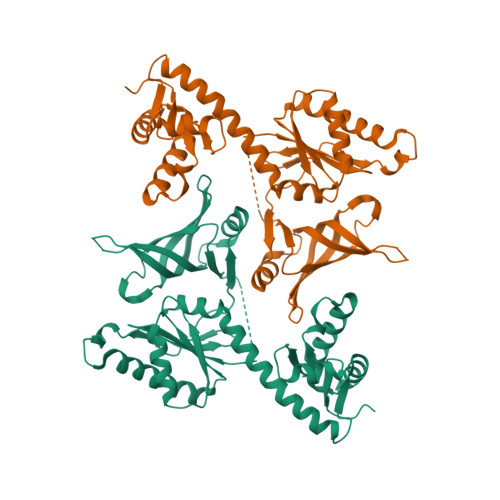
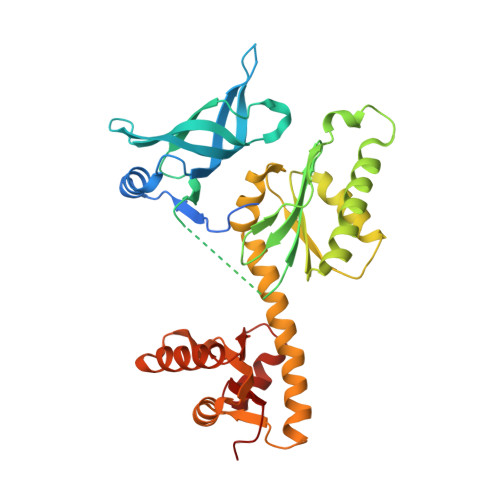
Crystal structures of two archaeal Pelotas reveal inter-domain structural plasticity
Lee, H.H., Jang, J.Y., Yoon, H.-J., Kim, S.J., Suh, S.W.(2010) Biochem Biophys Res Commun 399: 600-606
- PubMed: 20682285
- DOI: https://doi.org/10.1016/j.bbrc.2010.07.121
- Primary Citation of Related Structures:
3OBW, 3OBY - PubMed Abstract:
Dom34 from Saccharomyces cerevisiae is one of the key players in no-go mRNA decay, a surveillance pathway by which an abnormal mRNA stalled during translation is degraded by an endonucleolytic cleavage. Its homologs called Pelota are found in other species. We showed previously that S. cerevisiae Dom34 (domain 1) has an endoribonuclease activity, which suggests its direct catalytic role in no-go decay. Pelota from Thermoplasma acidophilum and Dom34 from S. cerevisiae have been structurally characterized, revealing a tripartite architecture with a significant difference in their overall conformations. To gain further insights into structural plasticity of the Pelota proteins, we have determined the crystal structures of two archaeal Pelotas from Archaeoglobus fulgidus and Sulfolobus solfataricus. Despite the structural similarity of their individual domains to those of T. acidophilum Pelota and S. cerevisiae Dom34, their overall conformations are distinct from those of T. acidophilum Pelota and S. cerevisiae Dom34. Different overall conformations are due to conformational flexibility of the two linker regions between domains 1 and 2 and between domains 2 and 3. The observed inter-domain structural plasticity of Pelota proteins suggests that large conformational changes are essential for their functions.
Organizational Affiliation:
Department of Bio & Nano Chemistry, Kookmin University, Seoul 136-702, South Korea. hhlee@kookmin.ac.kr