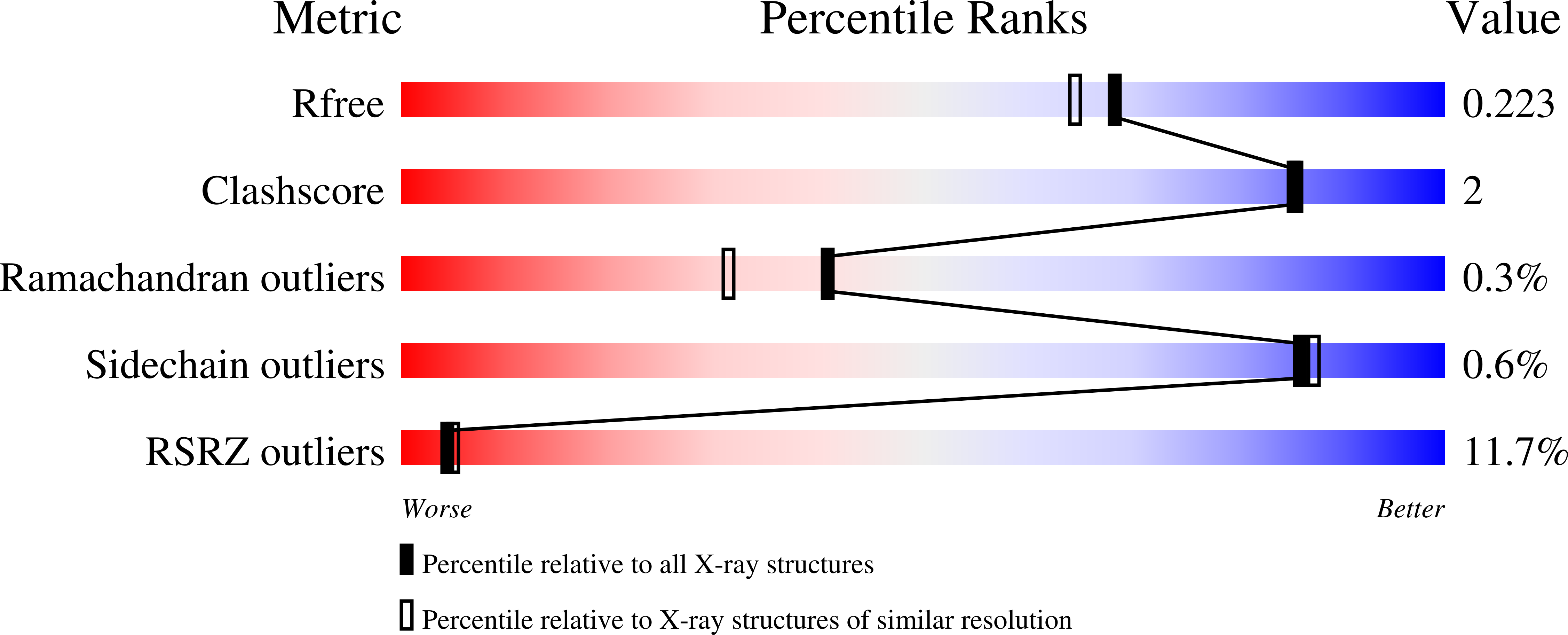
Structures of PGAM5 Provide Insight into Active Site Plasticity and Multimeric Assembly.
Chaikuad, A., Filippakopoulos, P., Marcsisin, S.R., Picaud, S., Schroder, M., Sekine, S., Ichijo, H., Engen, J.R., Takeda, K., Knapp, S.(2017) Structure 25: 1089-1099.e3
- PubMed: 28648608
- DOI: https://doi.org/10.1016/j.str.2017.05.020
- Primary Citation of Related Structures:
3MXO, 3O0T, 5MUF - PubMed Abstract:
PGAM5 is a mitochondrial membrane protein that functions as an atypical Ser/Thr phosphatase and is a regulator of oxidative stress response, necroptosis, and autophagy. Here we present several crystal structures of PGAM5 including the activating N-terminal regulatory sequences, providing a model for structural plasticity, dimerization of the catalytic domain, and the assembly into an enzymatically active dodecameric form. Oligomeric states observed in structures were supported by hydrogen exchange mass spectrometry, size-exclusion chromatography, and analytical ultracentrifugation experiments in solution. We report that the catalytically important N-terminal WDPNWD motif acts as a structural integrator assembling PGAM5 into a dodecamer, allosterically activating the phosphatase by promoting an ordering of the catalytic loop. Additionally the observed active site plasticity enabled visualization of essential conformational rearrangements of catalytic elements. The comprehensive biophysical characterization offers detailed structural models of this key mitochondrial phosphatase that has been associated with the development of diverse diseases.
Organizational Affiliation:
Institute for Pharmaceutical Chemistry, Johann Wolfgang Goethe-University and Buchmann Institute for Molecular Life Sciences, Max-von-Laue-Strasse 9, 60438 Frankfurt am Main, Germany; Nuffield Department of Clinical Medicine, Structural Genomics Consortium and Target Discovery Institute, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford OX3 7DQ, UK. Electronic address: chaikuad@pharmchem.uni-frankfurt.de.