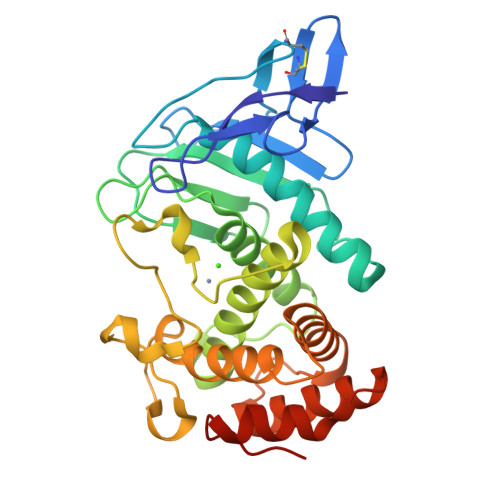
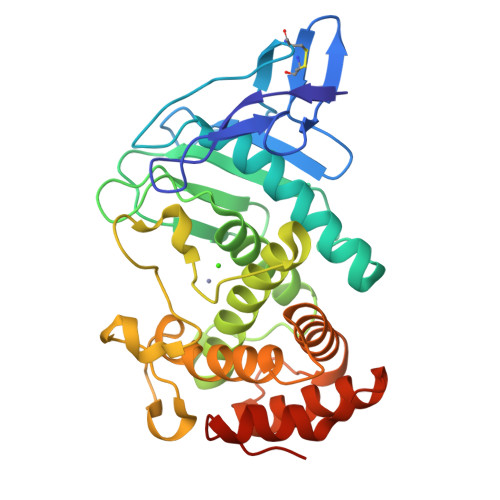
Structural basis for the autoprocessing of zinc metalloproteases in the thermolysin family
Gao, X., Wang, J., Yu, D.-Q., Bian, F., Xie, B.-B., Chen, X.-L., Zhou, B.-C., Lai, L.-H., Wang, Z.-X., Wu, J.-W., Zhang, Y.-Z.(2010) Proc Natl Acad Sci U S A 107: 17569-17574
- PubMed: 20876133
- DOI: https://doi.org/10.1073/pnas.1005681107
- Primary Citation of Related Structures:
3NQX, 3NQY, 3NQZ - PubMed Abstract:
Thermolysin-like proteases (TLPs), a large group of zinc metalloproteases, are synthesized as inactive precursors. TLPs with a long propeptide (∼200 residues) undergo maturation following autoprocessing through an elusive molecular mechanism. We report the first two crystal structures for the autoprocessed complexes of a typical TLP, MCP-02. In the autoprocessed complex, Ala205 shifts upward by 33 Å from the previously covalently linked residue, His204, indicating that, following autocleavage of the peptide bond between His204 and Ala205, a large conformational change from the zymogen to the autoprocessed complex occurs. The eight N-terminal residues (residues Ala205-Gly212) of the catalytic domain form a new β-strand, nestling into two other β-strands. Simultaneously, the apparent T(m) of the autoprocessed complex increases 20 °C compared to that of the zymogen. The stepwise degradation of the propeptide begins with two sequential cuttings at Ser49-Val50 and Gly57-Leu58, which lead to the disassembly of the propeptide and the formation of mature MCP-02. Our findings give new insights into the molecular mechanism of TLP maturation.
Organizational Affiliation:
State Key Laboratory of Microbial Technology, Marine Biotechnology Research Center, Shandong University, Jinan 250100, China.